Pentosidine, an Advanced Glycation End-Product, May Reflect Clinical and Morphological Features of Hand Osteoarthritis
Abstract
The study investigates pentosidine levels, an advanced glycation end-product, in patients with erosive and non-erosive hand osteoarthritis (HOA) and determine its potential association with clinical findings and imaging-defined joint damage.
Pentosidine was measured by HPLC in serum and urine of 53 females with HOA (31 erosive and 22 non-erosive HOA) and normalised to the total serum protein or urinary creatinine, respectively. Pain, joint stiffness and disability were assessed by the Australian/Canadian OA hand index (AUSCAN). The hand radiographs scored according to the Kallman grading scale were assessed to determine a baseline value and reassessed after two years.
The levels of urine pentosidine, but not of serum pentosidine, were higher in patients with erosive HOA than in non-erosive HOA (p=0.039). Urinary pentosidine correlated with CRP (r=0.302, p=0.031), ESR (r=0.288, p=0.041) and AUSCAN (r=0.408, p=0.003). Serum pentosidine, but not in urine, significantly correlated with the Kallman radiographic score in erosive HOA at the baseline (r=0.409, p=0.022) and after 2 years (r=0.385, p=0.032). However, when corrected for age and disease duration, only correlation between urine pentosidine and AUSCAN remained significant (r=0.397, p=0.004).
Our data suggest that serum and urine pentosidine levels may relate to the distinctive clinical and morphological features of HOA.
INTRODUCTION
Osteoarthritis (OA) is the most prevalent rheumatic joint disease, and it affects approximately 15% of the population [1]. The disease occurs and causes disability mostly among elders; however, hand OA (HOA) is also prevalent in middle-aged and post-menopausal women [2]. HOA is considered to be a subset of primary generalised nodal OA. HOA, particularly its erosive subset, is associated with substantial pain, stiffness and physical disability, which may be similar to those symptoms seen in patients with rheumatoid arthritis (RA) [3]. Erosive HOA is characterised by prominent local inflammation of hand joints, leading to central erosions of the distal and, less frequently, of the proximal interphalangeal joints [4]. Routine blood tests are not helpful, and no biomarker is currently of any specific value for monitoring or predicting OA in an individual patient.
The pathogenesis of OA is still unclear. However, the age-associated accumulation of advanced glycation end-products (AGEs) in joint tissues has been shown to contribute to molecular changes and pathologic alterations in articular cartilage, which then may be more susceptible to damage and development of OA [5-7]. Pentosidine was characterised as a sensitive marker for all AGEs and was demonstrated to have increased levels in the articular cartilage [7, 8]. In our previous study, we demonstrated the presence of increased serum levels of pentosidine in patients with knee OA in comparison to healthy controls, and we found a positive correlation between the levels of pentosidine and of cartilage oligomeric matrix protein (COMP), a marker of articular cartilage damage [9]. More recently, we also identified pentosidine as a potential marker with predictive value for radiographic joint damage in patients with knee OA [10].
Based on our previous data, we were curious to explore the association between pentosidine levels and hand OA. In the present study, we therefore investigated levels of serum and urine pentosidine in female patients with erosive and non-erosive HOA and characterised their potential association with clinical findings and radiographic joint damage over time.
MATERIALS AND METHODOLOGY
Patients
The patients in this study were selected from the group of patients who had undergone radiographs of both hands at a baseline and again after two years in our previous studies [11,12]. Altogether, 53 female patients that fulfilled the American College of Rheumatology (ACR) criteria for HOA [13] were included, 31 with the erosive and 22 with the non-erosive disease. The erosive disease was characterised according to the radiographic criteria published by Pattrick [14]. All patients were assessed for pain and effusion upon palpation, deformity and deviation. The Australian/Canadian OA hand index (AUSCAN) and Dreiser algo-functional index, a 10-item investigator-administered questionnaire, were evaluated [15, 16]. None of the patients had diabetes mellitus and/or renal disease that could influence the pentosidine levels. This study was designed in accordance with the Declaration of Helsinki (2000) of the World Medical Association and approved by the local ethical committee. All the participating patients signed informed consent. Their demographic data are summarised in Table 1.
Demographic and Clinical Characteristics of Patients with Erosive and Non-Erosive Hand Osteoarthritis (HOA)
| Characteristics | Erosive HOA (n=31) | Non-Erosive HOA (n=22) | p |
|---|---|---|---|
|
|
|||
| Age (years) | 65.55 ± 8.15 | 63.09 ± 10.85 | 0.551 |
| Sex (females/ males) | 31/0 | 22/0 | - |
| Disease duration (years) | 8.94 ± 7.74 | 7.16 ± 4.84 | 0.650 |
| CRP (mg/l) | 1.81 ± 1.15 | 1.79 ± 1.77 | 0.378 |
| ESR (mm/1st hour) | 13.26 ± 8.82 | 14.43 ± 9.10 | 0.530 |
| AUSCAN total | 41.55 ± 11.89 | 32.32 ± 10.17 | 0.003 |
| Dreiser algo-functional index | 18.45 ± 4.77 | 14.95 ± 4.67 | 0.005 |
|
|
|||
| Physical Examination (Mean Count of Affected Joints ± SD) | |||
| Deformity | 11.84 ± 4.12 | 8.32 ± 4.74 | 0.008 |
| Deviation | 4.32 ± 3.05 | 1.09 ± 1.95 | <0.001 |
| Tenderness | 9.16 ± 4.03 | 5.09 ± 3.31 | <0.001 |
| Effusion | 4.39 ± 4.15 | 1.68 ± 2.17 | 0.014 |
|
|
|||
| Radiographs | |||
| Baseline Kallman score | 58.90 ± 28.89 | 26.14 ± 18.57 | <0.001 |
| Kallman score at year 2 | 61.39 ± 28.67 | 26.73 ± 19.16 | <0.001 |
| Progression (difference from baseline to year 2) | 2.48 ± 3.13 | 0.59 ± 1.59 | 0.002 |
| Presence of knee OA (%) | 70.0 | 59.1 | 0.425 |
|
|
|||
| Three Phase Bone Scintigraphy | |||
| Blood pool (count of inflamed joints) | 3.90 ± 3.24 | 1.45 ± 2.14 | 0.002 |
| Late phase (count of remodelled joints) | 14.77 ± 6.28 | 6.00 ± 5.99 | <0.001 |
Legend: n = number of patients, p = statistical significance, CRP = C-reactive protein; ESR = Erythrocyte Sedimentation Rate, AUSCAN = Australian/Canadian Osteoarthritis Hand Index, HOA = hand osteoarthritis. The data are expressed as the mean ± SD, unless stated otherwise.
Association of Pentosidine, Clinical and Morphological Features of HOA
| r (before adjustment) | p (before adjustment) | Rho (after adjustment) | p (after adjustment) | |
|---|---|---|---|---|
|
|
||||
| Erosive HOA | ||||
| U-PEN and CRP | 0.117 | 0.539 | 0.132 | 0.502 |
| U-PEN and ESR | 0.217 | 0.240 | 0.151 | 0.444 |
| U-PEN and AUSCAN | 0.277 | 0.132 | 0.250 | 0.190 |
| U-PEN and K0 | 0.266 | 0.148 | 0.201 | 0.296 |
| U-PEN and K2 | 0.253 | 0.170 | 0.187 | 0.331 |
| S-PEN and CRP | -0.064 | 0.737 | -0.108 | 0.585 |
| S-PEN and ESR | -0.035 | 0.853 | -0.075 | 0.703 |
| S-PEN and AUSCAN | 0.325 | 0.075 | 0.242 | 0.206 |
| S-PEN and K0 | 0.409 | 0.022 | 0.297 | 0.118 |
| S-PEN and K2 | 0.385 | 0.032 | 0.276 | 0.147 |
|
|
||||
| Non-Erosive HOA | ||||
| U-PEN and CRP | 0.163 | 0.481 | 0.156 | 0.536 |
| U-PEN and ESR | 0.435 | 0.055 | 0.444 | 0.065 |
| U-PEN and AUSCAN | 0.303 | 0.183 | 0.139 | 0.571 |
| U-PEN and K0 | -0.414 | 0.062 | -0.261 | 0.281 |
| U-PEN and K2 | -0.422 | 0.057 | -0.277 | 0.251 |
| S-PEN and CRP | 0.131 | 0.572 | 0.157 | 0.534 |
| S-PEN and ESR | 0.143 | 0.536 | 0.009 | 0.972 |
| S-PEN and AUSCAN | -0.020 | 0.930 | -0.162 | 0.508 |
| S-PEN and K0 | -0.310 | 0.161 | -0.121 | 0.622 |
| S-PEN and K2 | -0.304 | 0.170 | -0.119 | 0.629 |
|
|
||||
| Erosive + Non-Erosive HOA | ||||
| U-PEN and CRP | 0.302 | 0.031 | 0.251 | 0.085 |
| U-PEN and ESR | 0.288 | 0.041 | 0.231 | 0.114 |
| U-PEN and AUSCAN | 0.408 | 0.003 | 0.397 | 0.004 |
| U-PEN and K0 | 0.173 | 0.219 | 0.227 | 0.114 |
| U-PEN and K2 | 0.166 | 0.238 | 0.211 | 0.141 |
| S-PEN and CRP | 0.010 | 0.946 | -0.066 | 0.657 |
| S-PEN and ESR | 0.037 | 0.031 | -0.019 | 0.897 |
| S-PEN and AUSCAN | 0.177 | 0.205 | 0.142 | 0.326 |
| S-PEN and K0 | 0.122 | 0.383 | 0.105 | 0.468 |
| S-PEN and K2 | 0.109 | 0.439 | 0.090 | 0.535 |
Legend: p = statistical significance, HOA = hand osteoarthritis; U-PEN = urinary pentosidine; S-PEN = serum pentosidine; CRP = C-reactive protein; ESR = Erythrocyte Sedimentation Rate; AUSCAN = Australian/Canadian Osteoarthritis Hand Index; K0 = Baseline Kallman score; K2 = Kallman score after 2 years; r = Spearman correlation coefficients (without adjustment to age and disease duration); rho = Spearman partial correlation coefficients corrected to age and disease duration.
Laboratory Analysis
Venous blood was drawn from all patients at the time of the urinary sample collection. Vacutainers with blood samples were centrifuged at 2000 rpm (600 g) for 10 minutes, and serum and urine samples were stored at –20°C until the time of analysis.
Pentosidine levels were measured in serum and urine samples by high performance liquid chromatography (HPLC) combined with sensitive fluorescent detection. Due to very low concentrations of pentosidine and complexity of the analyzed material, previous sample pretreatment was a necessary step. The samples were hydrolysed for 16 hours at 105°C in an aliquot of 35% HCl, the hydrolysate was purified and preconcentrated by solid phase extraction using the self-made purification columns filled with microgranular cellulose and the n-butyl alcohol/ acetic acid system was applied. The pentosidine-containing fraction was then desorbed with 0.05 mol/l hydrochloric acid, the extract was evaporated and the residue was reconstituted in a mobile phase containing 0.02 mol/l heptafluorobutyric acid, 0.01 mol/l ammonium sulphate and acetonitrile (linear gradient 12.5 – 25.0 % in 20 minutes). After this procedure the sample was applied into a thermostated (40°C) C18 reversed phase separation column (Separon SGX C18, 150x3 mm from Tessek; Prague, Czech Republic) of the HPLC system (Shimadzu, LC-10ADvp; Kyoto, Japan). The chromatographic system operated by the CLASS VP software enable the gradient flow of the mobile phase (flow rate 0.5 ml/ min.) and sensitive fluorescent detection (excitation/ emission wavelengths λ = 335/385 nm). Length of one chromatographic run was 30 minutes.
Pentosidine standard is not commercially available, so the synthetic pentosidine standard used for calibration was prepared in our laboratory by a modified version of the procedure kindly provided by Prof. V. M. Monnier (Case Western Reserve University, Cleveland, OH, USA) which is based on the unique polymer-analogical non-enzymatic reaction (using polymer form of lysine and the proper carbohydrates). The quantity and purity of the prepared standard was assessed by Dr. D. Sell (the same affiliation). A detailed description of the method was published earlier [17, 18].
The urine pentosidine (U-PEN) levels were normalised to the creatinine levels to account for differences in urine concentrations, and the serum pentosidine (S-PEN) levels were normalised to the levels of total serum protein. The high sensitivity C-reactive protein (hsCRP), total serum protein and urinary creatinine levels were measured by standard biochemical methods using the biochemical analyser Olympus (model AU 400, Japan).
Imaging
Each patient underwent posteroanterior plain radiographs of both hands at a baseline period and again after two years. Radiographs were scored by a trained reader according to the Kallman grading scale [19]. Individual joints were assessed for the presence of osteophytes (graded 0-3), joint space narrowing (0-3), subchondral sclerosis (0-1), subchondral cysts (0-1), lateral deformity (0-1), and the collapse of a central joint cortical bone (0-1). To exclude the impact of knee OA on the levels of serum pentosidine, radiographs of both knees were performed to obtain baseline images.
To evaluate the inflamed hand joints and bone remodeling, blood pool (second phase) images and late (third phase) images of the three-phase bone scintigraphy were performed following a bolus injection of 600 MBq of Tc-99m methylene disphosphonate into an antecubital vein. All of the planar anterior images that were focused on the small hand joints were interpreted by the same radiologist. Hand joints were considered inflamed when the blood pool images indicated increased indicator uptake. Osteoblastic activity representing bone remodeling was established by increased indicator uptake in the late phase. For the purpose of this study, the total number of positive joints was calculated.
Statistical Analysis
Statistical analysis was done using GraphPad Prism 5 (GraphPad Prism Software Inc.) and SAS statistical software (version 9.2; www.sas.com). For comparison between the two groups, Mann-Whitney test was used. The results were expressed as the mean ± standard deviation (SD). Significance level 0.05 was used. Nonparametric analysis of correlations was performed, when variables were not normally distributed, using Spearman correlation coefficient (r) or partial Spearman correlation coefficients (rho). Multivariate correlation analysis of dependence between variables of interest (U-PEN, S-PEN, Kallman scores, CRP, ESR) was performed, and partial Spearman correlation coefficients were computed, adjusted for both age and disease duration.
RESULTS
This study was designed to particularly recruit patients with erosive HOA; therefore more patients with the erosive disease than with the non-erosive disease were enrolled in this study. The patients in the two groups did not significantly differ in terms of age, sex, disease duration, acute phase reactants or the presence of knee OA (Table 1). However, patients with the erosive disease used more non-steroidal anti-inflammatory drugs (NSAIDs), had more tender and swollen joints, had more deformities and deviations, and had more joint damage and inflammation as assessed by hand radiographs and scintigraphy than the patients with the non-erosive disease (Table 1).
Pentosidine and Clinical Findings
The levels of serum pentosidine that were normalised to the total protein content in patients with erosive HOA were comparable to those in patients with the non-erosive disease (1.06±0.61 vs 0.95±0.34 nmol/g; p=0.678) as shown in Fig. (1A). On the other hand, the urinary levels of pentosidine that were normalised to the urinary creatinine concentrations were significantly higher in erosive than in non-erosive HOA (2.69±1.66 vs 2.42±1.90 nmol/mmol, p=0.039) as shown in Fig. 1B). Using univariate analysis, in all patients with HOA, we found a significant correlation between the levels of serum and urinary pentosidine (r=0.343, p=0.013). The levels of urinary, but not serum, pentosidine correlated significantly with both CRP (r=0.302, p=0.031) and ESR (r=0.288, p=0.041). While the serum levels of pentosidine did not correlate with the measures of disease activity, pain, stiffness or number of swollen joints, the levels of urine pentosidine significantly correlated with the dimensions of joint pain, stiffness and disability as assessed by AUSCAN (r=0.408, p=0.003). However, the urine pentosidine levels did not correlate with the Dreiser algo-functional index. A trend of association between the urinary pentosidine levels and the total number of tender joints (r=0.269, p=0.054), but not with the number of swollen joints (r=-0.142, p=0.316) was observed. When adjusted for age and disease duration, correlation between urine pentosidine and AUSCAN remained significant (r=0.397, p=0.004) (Table 2).
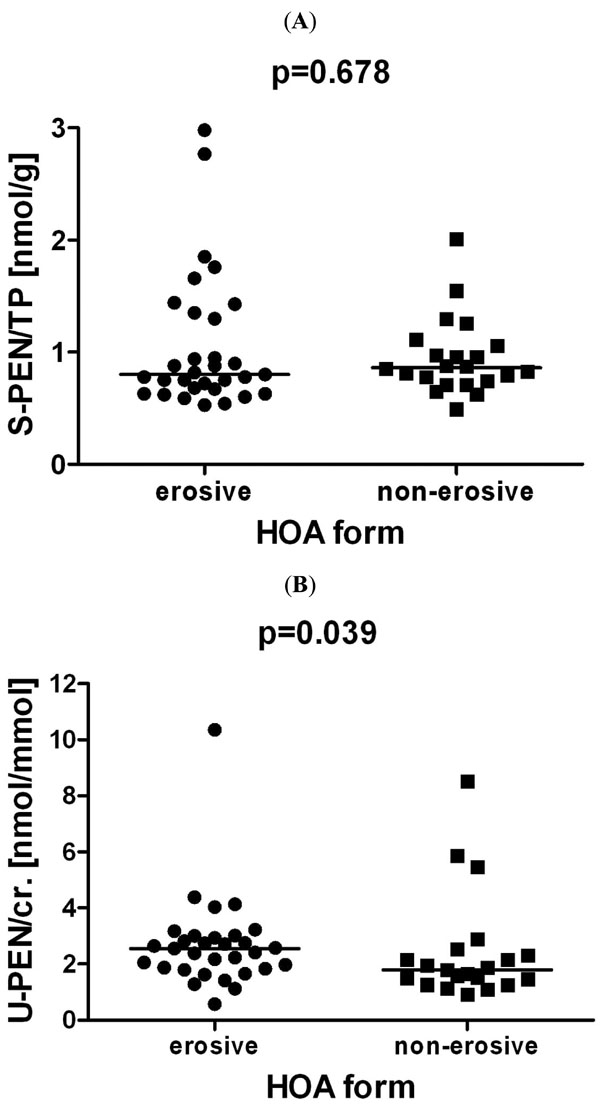
Serum (A) and urinary pentosidine (B) in patients with erosive and non-erosive hand osteoarthritis (HOA). The serum pentosidine levels were normalised to the levels of total serum protein (S-PEN/TP), and the urine pentosidine levels were normalised to the creatinine levels (U-PEN/cr.). The horizontal lines represent the median value.
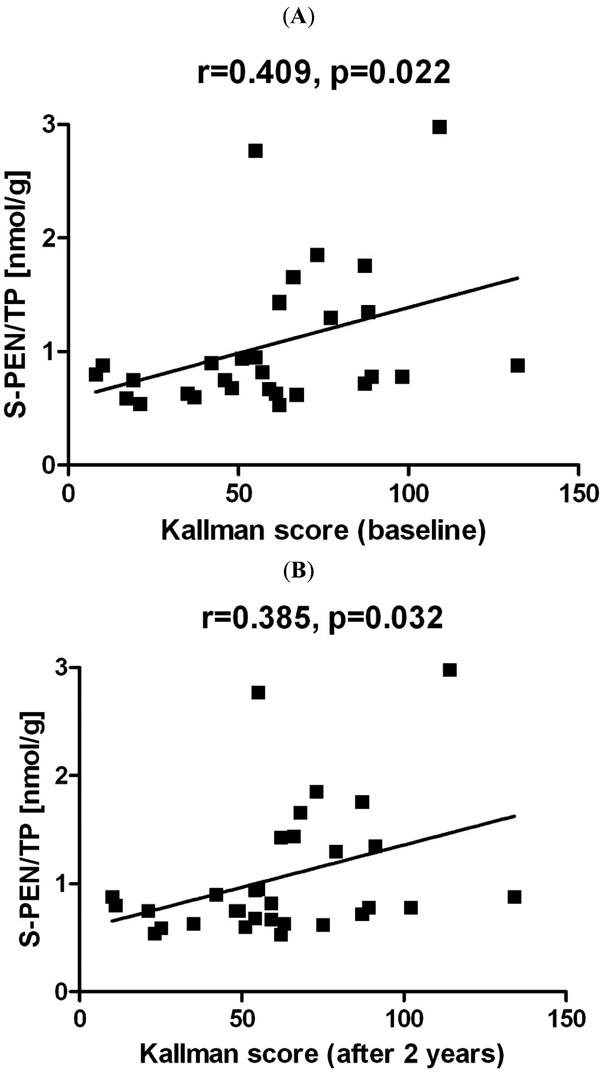
Correlations of S-PEN with Kallman radiographic scores in HOA: (A) baseline, (B) after two years. The shown graphs were constructed from the data without adjustment to age and disease duration. Abbreviations: S-PEN/TP = serum pentosidine levels normalised to the levels of total serum protein; HOA = hand osteoarthritis; r = Spearman correlation coefficient; p = statistical significance.
Pentosidine and Structural Joint Damage
Patients with the erosive disease had more severely affected hand joints, as assessed by both radiographs and scintigraphy, than those with the non-erosive disease (Table 1). As expected, the mean radiographic progression and scintigraphic progression over a period of two years were much faster with the erosive than with the non-erosive disease (Table 1). In all patients with HOA, the levels of serum and urine pentosidine did not correlate with the baseline joint radiographic damage (r=0.122, p=0.383 and r=0.173, p=0.219; respectively) or with the damage observed after two years (r=0.109, p=0.439 and r=0.166, p=0.238; respectively). However, in a subgroup of patients with the erosive disease, the levels of serum pentosidine significantly correlated with the Kallman baseline radiographic score and with the score determined after 2 years (r=0.409, p=0.022 and r=0.385, p=0.032; respectively) as shown in Fig. (2). On the contrary, urinary pentosidine levels did not correlate with the baseline Kallman radiographic score or with the radiographic score determined after 2 years (r=0.266, p=0.148 and r=0.253, p=0.170; respectively), and this association was also not found in patients with the non-erosive disease in which little progression was seen. When corrected for age and disease duration, correlation between serum pentosidine and radiographic scores remained no longer significant (Table 2). The levels of serum and urinary pentosidine in both groups of patients were not associated with the number of joints positive for blood pool phase and late phase scintigraphy (data not shown).
DISCUSSION
In the present study, we demonstrate for the first time higher levels of urine pentosidine, but not of serum pentosidine, in patients with erosive than in those with non-erosive hand OA. Additionally, the urine levels of pentosidine corrected for age and disease duration correlated significantly with assessments of joint pain, stiffness and disability in patients with HOA.
The levels of pentosidine were previously shown to be increased mostly under the conditions of inflammation, and they were associated with the laboratory and clinical disease activity in patients with RA [8, 20, 21]. Patients with OA have significantly lower levels of urine pentosidine than those with RA [20]. Although the levels of CRP were within the normal range in both groups of HOA patients, we found a positive correlation between the urinary pentosidine levels and acute phase reactants in patients with HOA. However, we did not observe this association with the levels of serum pentosidine, which is concurrent with two recent studies showing that the levels of serum pentosidine and other AGEs do not always reflect the disease activity in patients with RA [22, 23]. Our data also show that the levels of urinary pentosidine are related to the measurements of joint pain, stiffness and disability that were assessed by AUSCAN in patients with HOA. On the other hand, the levels of urinary pentosidine did not correlate with the degree of radiographic changes of finger joints. This may be consistent with recent data showing that urinary pentosidine levels do not correlate with radiographic changes in large joints [24] and do not predict knee cartilage loss in patients with knee OA [25].
The non-enzymatic glycation of proteins such as collagen or aggrecan in articular cartilage contributes to the formation of AGEs. This process is associated with ageing and results in increased cartilage stiffness, increased degradation of extracellular matrix proteins and decreased proteoglycan synthesis by chondrocytes [5]. Even though the presence of AGEs in articular cartilage is not specifically related to OA, the age-related accumulation of AGEs in the cartilage is likely to play a role in the pathogenesis of the disease [5-7, 26]. Pentosidine represents an adequate marker for all AGEs, and it is found in articular cartilage, serum, urine and synovial fluid [7-10, 17, 18, 20-22, 25]. Recently, urinary pentosidine levels were found to be elevated in patients with knee and hip OA in comparison to healthy control group individuals [18]. We also reported elevated levels of serum pentosidine in patients with knee OA and an association between the levels of pentosidine, the local cartilage degrading marker COMP and radiographic knee joint damage [9, 10]. Furthermore, the non-enzymatic glycation of collagen type I and the accumulation of pentosidine are also associated with osteoporosis and can induce the deterioration of bone quality [27]. Although we found no difference in the levels of serum pentosidine between patients with erosive and non-erosive HOA, we observed a significant association between the levels of serum pentosidine and radiographic changes in patients with the erosive disease. It can be therefore hypothesised that the levels of pentosidine may reflect the progressive bone and cartilage damage in erosive HOA. In that sense, our results may be similar to the recent finding of the C-terminal cross-linking telopeptides of type I collagen being correlated with the radiographic changes in patients with erosive HOA [28]. Because systemically measured pentosidine and other circulating biomarkers reflect whole body cartilage or bone degradation, they may be related to the severity of all joints affected by OA. Moreover, when the serum pentosidine was corrected for age and disease duration, its association with the degree of joint damage was no longer significant. These limitations have to be considered while interpreting the results of this and other studies.
CONCLUSIONS
In conclusion, the results of this study demonstrated increased levels of urine pentosidine in patients with erosive hand OA in comparison to those with non-erosive disease and suggested that the urine pentosidine is related to subclinical inflammation and measures of joint pain, stiffness and disability in patients with hand OA. Further studies are necessary to show whether pentosidine can be used as a surrogate marker of severity in age-related diseases such as OA.
ACKNOWLEDGEMENT
The study was supported by The Ministry of Health of The Czech Republic – research project No. 00023728.
CONFLICT OF INTEREST
The authors declare there is no conflict of interest.


