All published articles of this journal are available on ScienceDirect.
PERFUSE - A Real-World Study on Rheumatology Patients’ Persistence with Adalimumab Biosimilar SB5 in France: Impacts of Patient Experience
Abstract
Aim
This study aimed to provide new insights into patient training and satisfaction using Patient-reported Outcome Measures (PROMs) and their impact on 12-month persistence using SB5 in France.
Background
SB5 is an EMA-approved adalimumab biosimilar, demonstrating bioequivalence, equivalent efficacy, and similar safety and immunogenicity as the reference biologic.
Objective
The purpose of this study was to assess the effect of training practices on SB5 use in rheumatology and patient satisfaction, and measure their impact on 12-month persistence of SB5 use in real life in France.
Methods
508 eligible patients diagnosed with rheumatoid arthritis (n=117), psoriatic arthritis (n=78), or ankylosing spondylitis (n=313) were included in the observational PERFUSE study between October 2018 and December 2020 at 25 clinical sites across France. PROMs were collected 1-month after baseline via an online questionnaire designed with patient associations’ input. Persistence of SB5 use was captured during routine visits. The study (clinical trial NCT03662919) received regulatory approval from French authorities on March 21, 2018.
Results
Training on the correct use of SB5 injections was accepted (naive = 92.4%; pretreated = 82.6%) and appreciated (naive = 95.9%; pretreated = 97.4%) by patients. Satisfaction scores were high for all subgroups. Higher satisfaction with the injection device was linked to a lower probability of discontinuing treatment [HR=0.87; 95% CI (0.79; 0.96); p<0.05], as was a worse perception of the illness assessed using the B-IPQ [HR=1.03; (1.00; 1.05); p<0.05]. Moreover, a significantly higher proportion of ePRO (electronic Patient-reported Outcomes) respondents (n=221/508) remained on SB5 at 12 months than non-respondents [66.4% (59.3; 72.5) vs. 48.7% (42.4; 54.8); p<0.05].
Conclusion
Lower initial satisfaction may serve as a useful indicator for identifying individuals at a higher risk of non-persistence. This could provide a basis for recommending the standardization of patient information practices throughout France, particularly for pretreated patients.
1. INTRODUCTION
Inflammatory Rheumatic Diseases (IRDs), such as Rheumatoid Arthritis (RA), Ankylosing Spondylitis (AS), and Psoriatic Arthritis (PsA) affect between 0.2 and 1.0% of the population, with RA being the most common of the three, accounting for a considerable clinical and socio- economic burden [1-5]. These pathologies progressively affect patients’ joints and bone structure, resulting in pain, reduced mobility, and difficulty in performing everyday tasks [6-8].
Treatments have evolved over the past decades, with the development of biologics designed to specifically target key factors in pathogenic mechanisms [9], such as Tumor Necrosis Factor (TNF), to limit the production of proinflammatory cytokines [10]. Since the first such drug (infliximab) [11, 12], other biologics have been developed and approved for commercial use, such as Adalimumab (ADL) [13]. As reference products came off patent, biosimilars were developed; these are now approved as equivalent to their reference biologics regarding their physicochemical properties, biological activity, and clinical efficacy, with similar safety and immunogenicity profiles [14]. ADL biosimilars were first made available for prescription in 2017 [15].
As biosimilars are more affordable than reference biologics, they enable the treatment of more patients within the same budget and thus have the potential to improve patient access to targeted therapies [16, 17]. Biosimilars may be perceived negatively compared to reference biologics, as their lower price is sometimes erroneously associated with lower quality [18]. Thus, physicians need to reassure their patients when changing their treatment to a biosimilar in order to reduce the risk of the nocebo effect (i.e., the appearance of a negative outcome due to an erroneous belief that the intervention will cause harm) [19, 20]. Indeed, providing a good patient experience is a key differentiating factor between clinically similar drugs [21]. This is why Patient-reported Outcome Measures (PROMs), which provide key information from the patients’ perspective [22, 23], are increasingly adopted and popular. PROMs can provide key insights into the patients’ perspective, e.g., RA care, daily life, treatment satisfaction, and well-being [24, 25], which are often unavailable in the case of conventional data collection methods (e.g. medical visits at investigation sites), generally resulting in the sparsity of available information [26].
Although clinical equivalence has been demonstrated in the development phase of biosimilars, new data on long-term safety and effectiveness, especially in indications other than the one studied in the registrational trial, serving the authorization process, are welcomed by authorities and clinicians.
The PERFUSE study was designed to address the need for real-world evidence involving patients routinely treated with SB2 or SB5 in France, being a longitudinal observational study on long-term persistence, effective- ness, and safety, designed with patient experience in mind, and including 914 patients who were receiving SB5, an EMA-approved ADL biosimilar, as routine therapy for their diagnosed rheumatic or Inflammatory Bowel Disease (IBD). Clinical data from routine specialist visits were reported by physicians for 12 months, and patient-reported data were collected using an online question- naire. To ensure their pertinence, questions were co-designed with patient associations.
In this analysis, the aim was to describe patient satisfaction of the rheumatology cohort treated with SB5 either as their first biologic therapy or switched from prior therapy, utilizing patient-reported data to assess their impact on 12-month persistence with SB5. We have presented the data reported by the patients concerning their illness, satisfaction, and confidence regarding their treatment, and their perception of their disease and medicine in general.
2. MATERIALS AND METHODS
2.1. Study Design
PERFUSE (NCT03662919) was a non-interventional, multicenter cohort study of patients with IBD or rheumatic Immune-mediated Inflammatory Disease (IMID) using ADL-biosimilar SB5, conducted from 19 June 2019 (first patient in) to 15 March 2022 (last patient out).
The primary outcome measure of the PERFUSE study was SB5 treatment persistence from baseline to month 12, as reported by physicians. Secondary outcomes included a description of patients’ satisfaction with and confidence in using SB5, perception of their interactions with physicians, beliefs about medicine, and perception of their illness as well as the effects of these patient-reported outcomes on SB5 treatment persistence. The database extract for this analysis was taken on 04 April 2022.
| Outcome Measures | Questionnaire/tool | Assessment |
|---|---|---|
| Primary outcome | ||
| SB5 treatment persistence | Reported during routine visits by site staff | The proportion of patients who were treated with SB5 at 12 months out of the total number of included eligible patients |
| Secondary outcomes | ||
| Patients’ satisfaction with and confidence in using SB5 | Ad hoc questions designed in collaboration with patient associations (supplementary material 1) | Higher scores indicated a higher degree of satisfaction or confidence (scale of 0 to 10) |
| Perception of patients regarding their interactions with their physician | Perceived Efficacy in Patient-Physician Interactions (PEPPI) questionnaire [28] | Higher scores (ranging from 0 to 50) indicated higher perceived efficacy |
| Beliefs about medicine | Beliefs about Medicines Questionnaire (BMQ) [29] | BMQ-general score (ranging from 4 to 20) assessed harm and overuse of medication in general (the higher the scores, the stronger the opinions about the harmfulness of drugs) BMQ-specific score (ranging from -20 to 20) evaluated the treatment and necessity to take it (score s< 0 indicated concerns to outweigh the necessity) |
| Perception of patients regarding their illness | Brief Illness Perception Questionnaire (B-IPQ) [34] | A higher score (ranging from 0 to 80) indicated a worse perception of patients regarding their illness |
The PERFUSE study included both a gastroenterology and a rheumatology cohort. These cohorts were analyzed separately as each specialty has different care practices in France, which could lead to different patient experiences. Regardless, an analysis of satisfaction and persistence was performed with the same methodology for both cohorts to facilitate comparability. Results for the gastroenterology cohort were presented by Bouhnik et al. (2023) [27].
To be included in the SB5 rheumatic cohort of the PERFUSE study, adult patients had to meet the following criteria: i) 18 years of age or older, ii) a clinical diagnosis of RA, AS, or PsA, iii) able to understand the information provided and complete French language questionnaires, and iv) initiated SB5 treatment between October 2018 and October 2020. Exclusion criteria for this study were: i) SB5 treatment for any other primary indication, ii) inability to attend regular check-ups, iii) inability to be followed by the same site for the study’s duration, or iv) pregnancy or expected pregnancy during the follow-up period.
2.2. Data Collection
Data collection methods and outcome measures are presented in Table 1.
Patient persistence with SB5 treatment was tracked during routine visits by site staff and data were recorded using an electronic Case Report Form (e-CRF). Data collection points included the baseline (month 0; M0=SB5 initiation), month 6 (M6=4-8 months), and month 12 (M12=10-15 months) post-SB5-initiation.
The electronic Patient-reported Outcomes (ePRO) questionnaire assessed the following dimensions of patient experience: patient satisfaction with and confidence in using SB5, perception of their interactions with physicians, beliefs about medicine, and perception of their illness. Validated questionnaires were used where available. Scores were calculated using the appropriate methods. The questionnaires were completed within one month of SB5 initiation treatment.
Both eCRF and ePRO were accessible via a secure web portal specific to the study. These data were collected from October 2018 to December 2020.
2.2.1. Patient Persistence with SB5
Persistence was defined as the proportion of patients who were still treated with SB5 at month 12. The number of patients still treated with SB5 was obtained from the information recorded in the eCRF by medical staff at each contact (patients' continued use of SB5 and, if applicable, date and reason of treatment discontinuation).
2.2.2. Patient Satisfaction and Confidence
Patient satisfaction was evaluated by bespoke questions designed in collaboration with patient associations (Supplementary material 1). This PROM was rated on a scale of 0 (worst) to 10 (best), with higher scores indicating a higher degree of satisfaction with or confidence in the treatment. These scores were used to evaluate patients’ satisfaction with the information received on biosimilars, confidence in their ability to use the injection device, satisfaction with the injection device (either prefilled syringe or auto-injector pen), and satisfaction with their overall care at the time of prescription.
2.2.3. Patient-physician Interactions
The French version of the Perceived Efficacy in Patient-Physician Interactions (PEPPI-5 from 0-50) [28] questionnaire was used to evaluate the quality of the dialogue between patients and their physicians at the time of prescription of SB5. PEPPI scoring was described by Maly et al. (1998), which ranges from 0 to 50, with higher scores indicating higher perceived self-efficacy in patient-physician interactions [29].
2.2.4. Patient Beliefs about Medicine
The French version of the Beliefs about Medicines Questionnaire (BMQ) [30] was used to assess patients’ beliefs, attitudes, and fears about their treatment and their illness. Fall et al. described scoring for the French version of the BMQ, which is composed of 2 subscores. The BMQ-specific score (range: -20 to 20) includes questions related to the need for treatment and concerns related to prescribing (two subscales: Necessity and Concerns). Negative scores indicate that concerns outweigh necessity. The BMQ-general score (range: 4 to 20) is focused on the beliefs that patients develop about medicine in general (two subscales: Overuse and Harm). Higher scores indicate positive beliefs about treatment [30, 31]. The BMQ is also utilized to detect and identify issues related to patient adherence [32, 33].
2.2.5. Patient Perception of their Illness
The French version of the Brief Illness Perception Questionnaire (B-IPQ) [34] was used to determine patients’ perception of their illness (ranging from 0 to 80) with low scores denoting a strong negative perception and high scores indicating a strong positive perception. Broadbent et al. (2005) described the scoring of the B-IPQ, which is divided into 8 subscores and summarized by an overall score. These subscores exhibit a linear relation- ship, meaning that they progressively increase in magnitude as they reflect the measured dimension more prominently [35]. A higher total score indicates a more positive view of the patient’s situation regarding their illness.
2.3. Statistical Analysis
ePRO data were analyzed only for patients who completed the ePRO and had received at least one dose of SB5 at the time of ePRO completion. The cohort was subdivided into two groups based on whether the patient had received a Biologic Subcutaneous (BSC) treatment prior to initiation of SB5, as the expected evolution of naive and switched patients are quite different in terms of disease history and activity, as well as beliefs.
2.3.1. General Analysis
All statistical analyses were performed using SAS software version 9.1 or later (SAS Institute Inc. SAS Campus Drive, Cary, North Carolina 27513, USA). Baseline qualitative characteristics were expressed in n (%). A Shapiro-Wilk test was performed to assess the normality of the distribution of quantitative variables. All normally distributed quantitative data have been reported using the mean value and Standard Deviation (SD) for each subgroup for whom data were available. Non-normally distributed data have been presented using the median value and Interquartile Range (IQR). Statistical differences between subgroups were computed using an Analysis of Variance (ANOVA) test for normally distributed quantitative variables and a non-parametric Mann-Whitney-Wilcoxon test for non-normally distributed variables. Significant differences have been shown using 95% Confidence Intervals (CIs) and computed using the p-value; p<0.05 was considered statistically significant. Otherwise, the term ‘n.s.’ for 'not significant’ was used to report no statistical difference between the groups.
2.3.2. Persistence Estimation and Correlation with PROMs
Persistence was evaluated using a Kaplan Meier (KM) approach. Patients for whom no persistence data were available at month 12 were censored for the purposes of the KM analysis (i.e., they were considered neither having stopped nor having maintained treatment).
Statistically significant differences between defined subgroups were estimated using a log-rank test and are denoted with a star in the relevant figure. Defined subgroups were (i) BSC-naive and BSC pretreated patients and (ii) ePRO respondents and ePRO non-respondents.
A univariate correlation analysis using a Cox model was performed to analyze the impact of quantitative variables (PEPPI score, satisfaction, BMQ, B-IPQ score) on persistence; results have been presented using the Hazard Ratio (HR) with associated 95% confidence interval and p-value.
2.4. Ethics Approval
This study’s methodology, data security, and scientific merit were reviewed and approved by the appropriate bodies.
The final amendment to the protocol for this study was approved by the competent national independent ethics committee (Comité de Protection des Personnes, CPP) in France on April 25th, 2019, in accordance with French regulations.
This study has conformed to all regulations concerning the use of personal data. All procedures have been carried out in accordance with the ethical rules and the principles of the Declaration of Helsinki and its later amendments.
3. RESULTS
3.1. Included Population and Response Rates
This study included 508 rheumatology patients (117 RA, 78 PsA, 313 AS) across 25 French sites. A description of the different populations is provided in Table 2. No statistically significant difference was found between the respondent and non-respondent populations in terms of baseline characteristics of age or disease duration, although the proportion of women among the respondent population was numerically higher. The included population comprised 223 BSC-naive patients and 285 BSC pretreated patients (Supplementary material 2).
Patient experience, satisfaction, and beliefs were assessed only for patients who completed the online questionnaire and who reported using SB5 at that time: 104 (46.6%) BSC-naive patients and 117 (41.1%) BSC pretreated patients (Supplementary material 2-3). Questionnaire response rates varied from site to site. Fig. (1a) shows a broad distribution, with some sites showing a zero-response rate, and others reaching 80%. On average, the response rate by site was 49.20% for pretreated patients and 41.33% for treated patients.
3.2. Patient Training Practices
The frequency at which training was offered to patients was assessed for all ePRO respondents, including those who had not started SB5 at the time of completing the questionnaire. Attendance and satisfaction were only assessed among those who had started SB5 at the time of completing the questionnaire.
Respondent BSC-naive patients reported being offered training on the proper use of the SB5 injection device 74.8% of the time (n=80/107), with a 92.4% (n=73/79) attendance rate (Fig. 1b and 1c). Training mostly occurred at the hospital (94.5%, n=69/73). The length of each training was deemed satisfactory (neither too short nor too long) by 95.9% (n=70/73) of patients who attended the training (Fig. 1c). Training rates for naive patients were inconsistent, with sites offering training to 72.0% ± 35.3 (SD) of the patients on average, though individual site rates ranged between 0 and 100% (Fig. 1b).
| Global Population |
BSC Naive [N = 223 (43.9%)] |
BSC Pretreated [N = 285 (56.1%)] |
Total (N = 508) |
|---|---|---|---|
| Respondent population (completed ePRO and was confirmed to be treated by SB5 at the time), n (%) | |||
| 104 (46.6%) | 117 (41.1%) | 221 (43.5%) | |
| Age in years, mean (SD) | |||
| Completed ePRO Did not complete ePRO |
47.6 (13.5) 47.0 (15.1) |
51.0 (12.8) 50.9 (15.0) |
49.4 (13.2) 49.3 (15.1) |
| Disease duration, mean (SD) | |||
| Completed ePRO Did not complete ePRO |
5.5 (7.7) 4.6 (6.8) |
13.4 (11.1) 13.6 (11.8) |
9.7 (10.4) 9.9 (10.9) |
| Number of women, n (% of each subgroup) | |||
| Completed ePRO Did not complete ePRO |
64 (59.3%) 61 (52.6%) |
63 (52.5%) 74 (44.8%) |
127 (57.5%) 134 (47.7%) |
| Level of education of respondents, n (% of each subgroup)* | |||
| Less than high school diploma High school diploma or equivalent Up to bachelor’s More than bachelor’s |
15 (14.4%) 37 (35.6%) 28 (26.9%) 24 (23.1%) |
11 (9.4%) 53 (45.3%) 24 (20.5%) 29 (24.8%) |
26 (11.8%) 90 (40.7%) 52 (23.5%) 53 (24.0%) |
* Data are available for the respondent population only; these data were collected from the ePRO.

Comparison of response rates and training offerings in BSC naive and BSC pretreated patients. For all graphs, BSC pretreated patients are indicated with light grey colour and BSC naive patients are represented using dark grey colour. a. Distribution of ePRO answer rates per site. No statistical differences were observed between BSC naive and BSC pretreated patients. b. Distribution of rates at which training was offered to BSC-naive and pretreated patients. BSC naive patients were offered training significantly more often than BSC pretreated patients. c. Rates at which patients attended training if it was offered and the satisfaction with training length. * p<0.05.
Training on the proper use of the SB5 injection device was offered to 40% (n=48/120) of BSC pretreated patients, with an 82.6% (n=38/46) attendance rate (Fig. 1b and 1c). Training sessions mostly occurred at the hospital (92.1%, n=35). The length of each training was deemed satisfactory by 97.4% (n=37/38) of the patients (Fig. 1c). Training rates for BSC pretreated patients were also inconsistent with each site offering training to 31.8% ± 33.0% of their patients on average, though individual site rates were between 0 and 100% (Fig. 1b).
The rate at which training was offered to BSC naive patients was significantly higher than for BSC pretreated patients (p < 0.05) (Fig. 1b). Satisfaction with the training length was not significantly different between the groups. The rate at which BSC pretreated patients accepted to take part in a training session when offered was numerically lower than for naive patients, although not statistically significant.
3.3. Patient Satisfaction and Confidence
Satisfaction with the information received on biosimilars was similarly high for both BSC naive and BSC pretreated patients (8.3 ± 1.5 and 8.1 ± 2.3, respectively; n.s.) as was patients’ confidence in their ability to use the injection device (8.0 ± 2.7 and 8.3 ± 2.6, respectively; n.s.), satisfaction with the injection device (8.0 ± 2.0 and 7.2 ± 2.2, respectively; n.s.), and satisfaction with their overall care at the time of prescription (8.1 ± 1.7 and 8.2 ± 1.9, respectively; n.s.) (Fig. 2a).
For both BSC naive and BSC pretreated patients, no statistically significant differences in terms of confidence in their ability to use the injection device (8.2 ± 2.5 and 8.2 ± 2.7, respectively; n.s.), satisfaction with the injection device (7.7 ± 1.9 and 7.4 ± 2.5, respectively; n.s.), or satisfaction with their overall care at the time of prescription (8.3 ± 1.4 and 8.0 ± 2.0, respectively; n.s.) were observed between patients who attended a training session on the proper use of the SB5 injection device (n=111) and those who did not (n=110) (Fig. 2a).
Similarly high satisfaction scores for both trained and untrained patients were observed in both BSC naive and BSC pretreated patients (Fig. 2a).
3.4. Patient-physician Communication
Patient-physician communication (PEPPI-5) was rated highly by both BSC naive and BSC pretreated patients, and no statistically significant difference was observed between the two groups (41.5 ± 6.1 and 42.1 ± 7.5, respectively; n.s.) or between patients who attended a training session and those who did not (41.7 ± 6.4 and 41.9 ± 7.3, respectively; n.s.) (Fig. 2b).
3.5. Patient Beliefs about Medicine
The BMQ specific necessity score (8.5 ± 2.6 and 8.5 ± 2.2; n.s) was significantly lower than the specific concern score (13.6 ± 3.3 and 14.4 ± 3.5; n.s) for BSC naive and BSC pretreated patients, respectively (p < 0.05 for both populations) (Fig. 3a). BMQ overall specific score was -5.1 ± 3.6 and -5.9 ± 3.8 for naive and pretreated patients, respectively (n.s.) (Fig. 3b).
The BMQ general harm (9.7± 2.5 and 9.8 ± 3.1; n.s) and overuse scores (10.2 ± 2.6 and 10.4 ± 3.1; n.s) were examined for BSC naive and BSC pretreated patients, respectively (Fig. 3a). Overall BMQ general scores were 19.9 ± 4.5 and 20.2 ± 5.7 for BSC naive and BSC pretreated patients, respectively (n.s.) (Fig. 3b), indicating that patients did not hold strong positive or negative beliefs about medicine in general.
3.6. Patient Perception of Illness
BSC naive patients had a slightly better view of their illness than BSC pretreated patients (B-IPQ scores of 47.6 ± 7.8 and 44.6 ± 10.2, respectively; p<0.05). However, scores remained moderate, indicating a generally ambivalent perception of their disease (Fig. 3c).
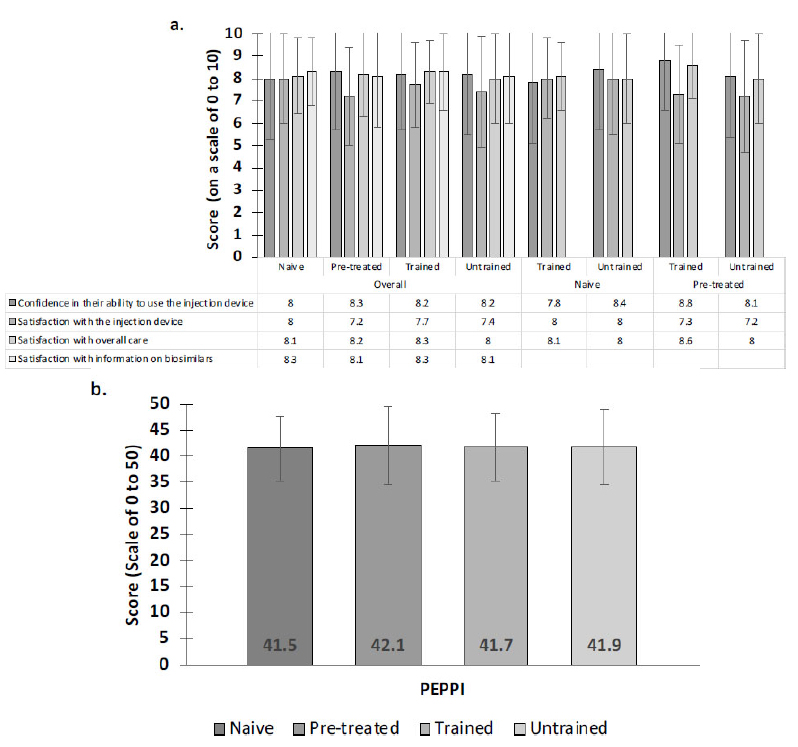
Satisfaction and confidence scores for each subgroup. a. Satisfaction and confidence scores were all high. No statistically significant differences were observed between any subgroups. b. The perceived efficacy of patient-physician dialogue was high overall. No significant differences were observed.
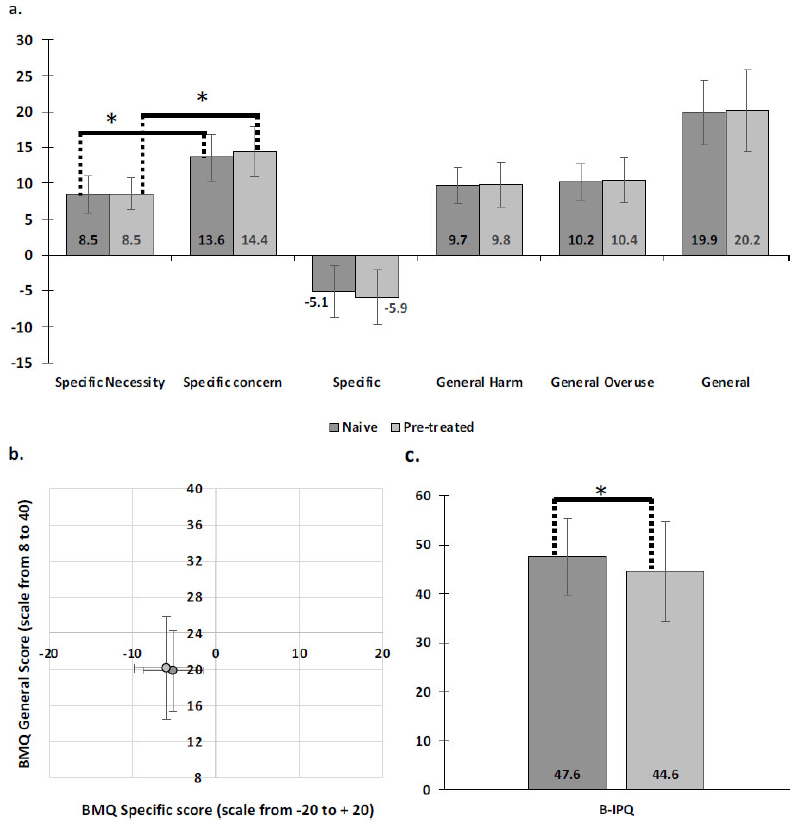
Patient beliefs and perception of their illness (scores). For all graphs, BSC pretreated patients are represented by light grey and BSC naive patients are represented by dark grey colour. a. BMQ scores. The specific concern score was significantly lower than the specific necessity score, indicating patients to be skeptical about their treatment. The general score was moderate. No differences were observed between the subgroups. b. Scatterplot of BMQ scores. Error bars indicate standard deviation. c. B-IPQ scores. This score was significantly higher for naive patients than for pretreated patients. * p < 0.05
3.7. Association between PROMs and Persistence with SB5
Persistence with SB5 at 12 months was numerically lower for trained than for untrained patients [65.5%, 95% CI (54.9; 74.1) and 68.9%, 95% CI (58.9; 77.0), respectively; n.s.] (Fig. 4A). However, persistence with SB5 at 12 months was significantly higher in respondents than in non-respondents [66.4% (59.3; 72.5) and 48.7% (42.4; 54.8), respectively; p<0.05] (Fig. 4B).
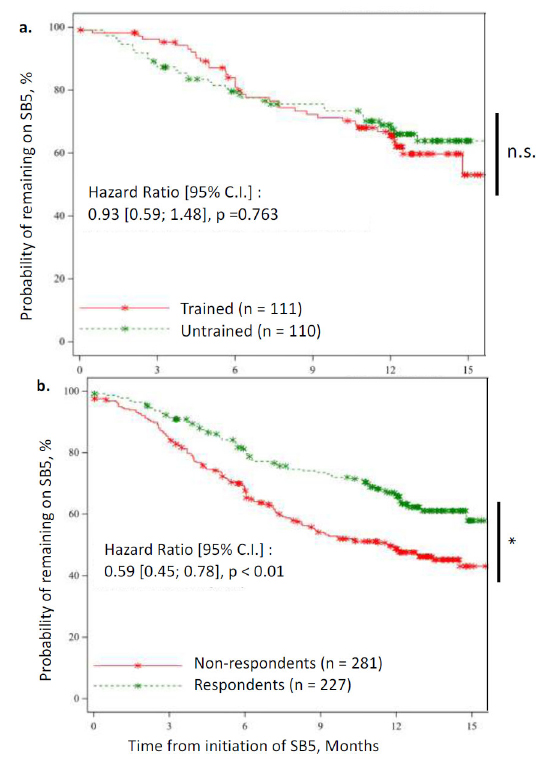
Kaplan-Meier survival curves for persistence of using SB5. Data are shown up to 15 months (end of M12 window). Persistence was calculated and compared at 12 months. Asterisks on the curve indicate censored patients and steps indicate a patient who stopped SB5. Statistical significance is shown on the right: * p < 0.05. a. Survival curves for trained patients (red) compared to untrained patients (green). No statistical difference was observed. b. Survival curves for non-respondents (red) compared to respondents (green). Persistence was significantly higher in respondents than in non-respondents.
A univariate Cox model analysis showed that patients who reported better satisfaction with the injection device were significantly less likely to stop treatment within the first year after SB5 initiation than those with lower satisfaction scores [HR=0.87 (0.79; 0.96), p<0.05]. This model also showed that patients with higher B-IPQ scores were more likely to stop treatment within this same period than those with lower scores [HR=1.03 (1.0;1.05), p<0.05]. Finally, no significant correlation with persistence was observed for PEPPI scores [HR=0.98 (0.95; 1.02), n.s.], BMQ general [HR=1.01 (0.97; 1.056), n.s.], or BMQ specific scores [HR=1.00 (0.94; 1.07), n.s.] (Table 3).
| Score | N | HR (95% CI) | p-value |
|---|---|---|---|
| PEPPI score | 221 | 0.982 (0.950; 1.015) | 0.2774 |
| Satisfaction with SB5 device | 221 | 0.869 (0.791; 0.955) | 0.0035* |
| BMQ specific | 221 | 1.000 (0.938; 1.067) | 0.9878 |
| BMQ general | 221 | 1.012 (0.969; 1.056) | 0.6017 |
| B-IPQ score | 221 | 1.025 (1.000; 1.050) | 0.0459* |
4. DISCUSSION
While most BSC naive patients were trained to use the injection device, most of the BSC pretreated patients were not. Numerically lower attendance rates for pretreated patients indicate that these patients felt training as not necessary, which has been reflected in higher confidence scores. While recommendations state that all patients receiving specific treatment for the first time should receive appropriate information and education on how to manage the new treatment [36, 37], we found specific training rates to be low overall. However, the reasons why training was not offered were not collected.
Results mirrored those observed in the gastro- enterology cohort, in which satisfaction levels and patient perceptions were similar. Indeed, these factors could be mainly related to the treatment itself, which was, however, the same for patients in both cohorts. However, results revealed contrasting training practices, with greater variability between rheumatology sites than between gastroenterology sites, and pretreated patients being trained more frequently by rheumatology sites than by gastroenterology sites. Additionally, the results of the correlation analysis were similar, with the key difference being the significance of the correlation between patient satisfaction and persistence [27].
Patient satisfaction with all assessed aspects of the prescription process was generally high, as has been observed in previous studies on biosimilars [38, 39]. Patients reported high levels of perceived efficacy in their dialogue with the prescribing physician and were generally highly satisfied with their experience at the time of prescription. No significant differences in terms of satisfaction were observed between BSC naive and BSC pretreated patients, nor between trained and untrained patients. This may indicate that neither prior experience nor specific patient training has a significant impact on any of the measured aspects of patient satisfaction when initiating or switching to SB5. It appears, therefore, that patient care at the time of the switch meets patients’ requirements without the need for separate specific training sessions. However, the reasons for switching to SB5, as well as the involvement of the patient in this decision, may constitute a potential bias in terms of treatment satisfaction and, consequently, persistence. Similarly, information on injection devices (i.e., whether a patient used syringes and auto-injector pens) was not collected. It is well-documented that auto-injector pens are generally better tolerated than syringes [40], which may have influenced patient satisfaction and experience.
Although patients’ beliefs about medicine in general were ambivalent, patients’ concerns about the potential adverse consequences of taking the study treatment outweighed the perceived necessity of using it to control their illness. This suggests that patient concern may principally stem from uncertainty surrounding the new treatment. Furthermore, patients’ beliefs about SB5 did not appear to be affected by their previous experience with a subcutaneous treatment. Indeed, pretreated patients had successfully been treated with a previous BSC and were thus less likely to have a negative preconception of SB5.
BSC naive patients had a significantly more positive view of their illness than BSC pretreated patients. Indeed, pretreated patients were living with their illness for much longer than BSC naive patients (Table 2) and were thus more likely to have experienced more flares. This is in line with previous studies, which have found disease duration to be negatively correlated with patients’ quality of life [41].
Patient training on the proper use of the injection device did not appear to be associated with persistence with SB5, either negatively or positively, and neither did BMQ nor PEPPI scores. These results seem to contradict previous findings that have reported enhanced communication strategies to lead to an increase in persistence [42, 43].
Respondents exhibited significantly higher persistence than non-respondents. This does not appear to be linked with patient status at inclusion, as the response rate was similar for both pretreated and naive patients, and, thus, may provide a useful tool to direct efforts to optimize persistence. This type of observation has been made in other therapeutic areas; cancer survival was observed to be better in patients who communicated via email with their physician [44], indicating that certain profiles of patients who are either more technologically inclined or more involved with their treatments may benefit more from them. Likewise, patients who are doing better may be more inclined to communicate.
However, although the association between B-IPQ score and persistence was statistically significant, the clinical significance was not clear. The analysis of factors affecting persistence was conducted using univariate regression, which may have introduced potential biases, such as limitations in capturing complex inter- relationships, confounding variables, and nonlinearity. Despite these limitations, the use of a multivariate analysis model was deemed unlikely to provide significant additional information compared to univariate analysis. This choice was justified by the observation that only one explanatory factor was clearly significant (satisfaction with the injection device; Table 3), while the remaining factors exhibited indications of multicollinearity.
Other limitations inherent to the design of the study and the type of data presented have been identified. First includes the relatively low response rate to the online questionnaire, which, at around 50%, was lower than we had hoped for, even though it appears to be in line with those observed in other studies using this delivery method (email along with email reminders) [45]. However, the reasons for non-response were unavailable; thus, the distribution of the participants was made by indication (RA, AS, or PsA), which may have led to potential biases. Second, large differences both in response and training rates were observed between the sites. To address this, reporting and training practices could be standardized to ensure training quality. However, this may be impossible for some investigation sites that do not have the necessary resources to provide proper patient training; this could possibly explain why some sites only trained a small percentage of patients or even none.
Additionally, pre-existing diagnoses, such as Juvenile Idiopathic Arthritis (JIA), which may have persisted to the adult age, may also affect patients’ experience with their ADL treatment according to some registries and surveys [46]. Patients with a primary diagnosis of JIA were excluded from this study in order to avoid this confusion factor [47]. However, the concomitant presence of other diseases that may be affected by ADL was not accounted for in the assessment of patient experience, as very few patients fell in this category (<1%).
The validated scores used in this study were chosen for their combination of simplicity (length and wording) and ease of interpretation. The bespoke satisfaction scores were constructed based on a commonly used scale for the evaluation of such measures. However, these questions have not been independently validated, and so their psychometric properties have not been assessed. Other possibilities for evaluating patient satisfaction exist, with the most common being 5 or 7-level Likert scales, as they are easily interpretable by the patient. We opted for a numerical scale, which could easily quantify the results. However, the interpretation of a numerical scale could be harder because patients’ understanding of the values within the scale may be different [48].
These ePRO results should be considered within the socio-economic context in which the data collection occurred, and which may have influenced patient-reported outcomes; this study was performed in France at a time when biologic and biosimilar treatments were at the forefront of debate in the health sector and patient associations were still working on providing accurate information about drug costs, funding, and reim- bursement. French patients have broad access to these treatments and are overall very well covered in terms of reimbursement for biologics. Thus, the economic benefits of biosimilar switching directly impact the healthcare system rather than the patients. The consensus among rheumatologists is that there is a need to promote the use of biosimilars in order to reduce expenses for the French public healthcare system [49], which makes the lack of training provided to pretreated patients all the more surprising.
CONCLUSION
This study has highlighted disparities in patient training practices, especially for pretreated patients, although this has not appeared to impact patient persistence with SB5 after 1 year. However, the study has indicated that patients who have expressed satisfaction with their treatment and those who have actively engaged in ePRO responses have tended to display greater levels of persistence. The satisfaction of patients could serve as a valuable marker to identify individuals more likely to exhibit non-persistence. Therefore, this finding suggests the need for standardized patient information practices across France, especially for pretreated patients.
AUTHORS’ CONTRIBUTION
It is hereby acknowledged that all authors have accepted responsibility for the manuscript's content and consented to its submission. They have meticulously reviewed all results and unanimously approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| ADL | = Adalimumab |
| ANOVA | = Analysis of variance |
| AS | = Ankylosing spondylitis |
| B-IPQ | = Brief Illness Perception Questionnaire |
| BMQ | = Beliefs about Medicines Questionnaire |
| BSC | = Biologic subcutaneous treatment |
| CI | = Confidence interval (95%) |
| e-CRF | = Electronic case report form |
| EMA | = European Medical Association |
| ePRO | = Electronic patient-reported outcome (e.g. online questionnaires) |
| HR | = Hazard ratio |
| IBD | = Inflammatory bowel disease |
| IMID | = Immune-mediated inflammatory disease |
| IQR | = Interquartile range |
| IRD | = Inflammatory rheumatic disease |
| JIA | = Juvenile idiopathic arthritis |
| KM | = Kaplan Meier |
| M0 | = Month 0 - baseline |
| M12 | = Month 12 |
| M6 | = Month 6 |
| n.s. | = Not (statistically) significant |
| PEPPI-5 | = Perceived Efficacy in Patient-Physician Interactions 5 Questions |
| PROM | = Patient-reported outcome measure |
| PsA | = Psoriatic arthritis |
| RA | = Rheumatoid arthritis |
| SD | = Standard deviation |
| TNF | = Tumor necrosis factor |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The final amendment to the protocol for this study was approved by an independent ethics committee (Comité de Protection des Personnes - CPP) in France, in accordance with French regulations, on April 25th, 2019.
This study is also listed on clinicaltrials.gov with the identifier NCT03662919 and was approved by the appropriate bodies in terms of the quality of the methodology, data security, and scientific merit.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
The information notice and consent form were provided to patients during a routine visit when patients were able to take as much time as needed to review the documents. The consent form (non-opposition, according to applicable French law), once signed, was archived in the study file. Only once this process was complete and eligibility confirmed could patients start participating in the study.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated or analyzed in the present study are not publicly available as data for this study contain potentially identifying information.
The code and functions used for analysis cannot be provided either as some of the functions used are proprietary and cannot be shared (property of eXYSTAT).
FUNDING
This study was funded by Biogen International GmbH (Baar, Switzerland). Funding for data management services, writing, and editorial support was provided by Biogen France SAS (Paris, France).
ACKNOWLEDGEMENTS
The authors acknowledge Sanoïa e-Health Services for data management services, writing, and editorial support for this manuscript as well as Ben Braithwaite and Arthur Bagel of Sanoïa e-Health Services for support in drafting and reviewing this manuscript. The authors also acknowledge eXYSTAT for data analysis services.


