RESEARCH ARTICLE
Oral Candidiasis in Patients with Rheumatoid Arthritis: A Hospital-Based Cohort Study
Cristhiane Almeida Leite da Silva1, 2, 3, *, Gabriela Camarneiro Siqueira3, Vander Fernandes1, 3, Luiz Evaristo Ricci Volpato1, 2, Walkiria Shimoya-Bittencourt1, 2, Alexandre Meireles Borba2, Bernar Monteiro Benites4, Ageo Mario Candido da Silva1
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e18743129259550
Publisher ID: e18743129259550
DOI: 10.2174/0118743129259550230922115211
Article History:
Received Date: 25/4/2023Revision Received Date: 15/8/2023
Acceptance Date: 25/8/2023
Electronic publication date: 10/11/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Rheumatoid (RA) arthritis is a chronic autoimmune disease characterized by symmetric polyarthritis and systemic inflammation. Systemic complications due to RA and its treatment may affect oral health.
Objective:
To assess the prevalence and predisposing factors for oral candidiasis in Rheumatoid Arthritis patients treated at a rheumatology referral hospital.
Methods:
This is a longitudinal study of a panel of repeated measures performed on patients attending the Rheumatology and Oral Diagnosis Outpatient Clinic of the General Hospital of Cuiabá. Patients were followed up between 2018 and 2019, with the information recorded in the medical records and subsequently extracted. A generalized estimation equation model was used to assess the association between oral candidiasis and RA.
Results:
In the final model, in crude (bivariate) analysis, the use of prednisone (OR 8.3) and tocilizumab (OR 3.7) were significantly associated with oral candidiasis. In the multivariate generalized estimation equation model, the use of prednisone (OR 9.9) and the presence of hyposalivation in male patients (OR 1.8) were the variables that remained statistically associated with oral candidiasis.
Conclusion:
The use of immunosuppressive drugs and the low rate of salivary flow in male patients increase the risk of oral candidiasis in patients with rheumatoid arthritis, reinforcing the importance of stomatological monitoring, a preventive approach and early diagnosis of this pathology.
1. INTRODUCTION
Rheumatoid arthritis (RA) is the most common systemic autoimmune disease, characterized by proliferative synovitis, and bone and cartilaginous tissue destruction, which can cause deformities, particularly involving the hand and wrist joints. It has a symmetrical, additive, inflammatory, and chronic character and unknown etiology. To make the diagnosis, in addition to the association of signs and symptoms, auxiliary laboratory and imaging tests are used [ 1, 2]. Drug treatment includes the use of nonsteroidal anti-inflammatory drugs, corticosteroids, and disease-modifying drugs (DMDs) to delay or interrupt the course of the disease, thus preventing the individual from having permanent sequelae [ 3].
Infectious complications are an important cause of increased mortality in this group of patients, and the reason for the increased susceptibility to infection is still unclear. However, factors such as the disease itself, the impact of chronic comorbid conditions, as well as sequelae of the treatment, could affect the immune system, predisposing the patient to infection [ 2, 4].
Although there is no specific oral manifestation of the disease, the drugs used for treatment may be associated with effects involving the oral mucosa, such as lichenoid reactions (associated with naproxen, infliximab, obinutuzumab, adalimumab, etanercept, and abatacept), ulcers (associated with ibuprofen, naproxen, and rofecoxib), hyposalivation, gingivitis, periodontitis, and other infections [ 5-9].
Oral candidiasis is an opportunistic fungal infection caused by Candida albicans, an opportunistic fungus that colonizes the surface of the oral mucosa and other mucous membranes such as the tracheobronchial, gastrointestinal, and genitourinary tracts [ 10, 11]. Local and systemic predisposing factors include the use of oral prostheses, hyposalivation, immunosuppression states, endocrine disorders, chemotherapy, radiation therapy, use of corticosteroids, immunomodulatory drugs, xerostomic drugs, and broad-spectrum antibiotics [ 12, 13]. It can be seen in conjunction with a hidden esophageal infection, and cases of disseminated infection can also originate from the oral mucosa [ 14].
The frequency of oral candidiasis amongst patients with RA, as are the variables associated with its occurrence, is poorly studied. Given the importance of prevention, early diagnosis, and effective treatment for infections in this group of patients, in combination with the scarcity of studies in this group of patients, this study aimed to determine and analyze the frequency of oral candidiasis and its association with demographic and clinical variables in a cohort of patients with RA.
2. MATERIALS AND METHODS
This is a prospective longitudinal survey of a panel of repeated measures, carried out at the Rheumatology and Oral Diagnosis Outpatient Clinic of the General Hospital of Cuiabá, Cuiabá, MT, Brazil. The study population consisted of patients with RA undergoing treatment who were followed up between January 2018 and December 2019. Patients were included according to their returns for follow-up, which varied according to the disease progression defined by the rheumatologist. The inclusion criteria were: having a diagnosis of RA according to the criteria established by the American College of Rheumatology [ 1] 18 years old or over, and presenting on the evaluated day with all variables necessary for the study. A diagnosis of more than one autoimmune rheumatic disease, including Sjogren Syndrome, was the sole exclusion criterion.
During the study period, all patients were followed up and evaluated by the research team and subsequently, the data were extracted from the medical records for analysis. All patients were submitted to a stomatological evaluation on the day of their consultation with the rheumatologist. In addition to oroscopy, cytopathological examination has been performed on the bilateral borders of the tongue and in all patients and unstimulated sialometry [15, 16]. In the absence of oral lesions, smears were collected only from the bilateral borders of the tongue for Papanicolaou staining and periodic acid-Schiff (PAS); in the presence of oral lesions suggestive of candidiasis, a smear was collected from the lesion area for PAS.
Papanicolaou staining, periodic acid-Schiff (PAS) technique and cytopathological analysis were performed by an oral pathology specialist. As a diagnostic criterion for oral candidiasis, the smears should reveal: the presence of hyphae/pseudo-hyphae associated with keratinised cells and inflammatory alterations ( i.e., nuclear hy-perchromatism, leukocytes, and perinuclear halo) [ 17].
For the descriptive study of the population, the following independent variables from the patients' first consultations were obtained: sex, age group (18–38, 39–59, ≥ 60 years), skin color (white, black, yellow, or brown), education (illiterate, incomplete or complete elementary school, incomplete or complete high school, incomplete or complete higher education), and provenance (municipality of origin). Data regarding the drugs administered (prednisone, methotrexate, leflunomide, chloroquine, abatacept, adalimumab, and tocilizumab), as registered by the rheumatologist, were extracted from the medical records. Hyposalivation was diagnosed when the unstimulated salivary flow rate was ≤ 0.3 mL/min over 5 min [15]. Oral candidiasis was diagnosed using oral exfoliative cytology and was considered as the dependent variable.
All patients involved in the research between January 2018 and December 2019 were selected by convenience according to their returns for follow-up, which varied according to the disease progression defined by the rheumatologist. All patients who underwent dental evaluation were included, still representing a number of participants.
Possible selection bias because they are exclusively patients from the public and not private network, which could represent lower education, and less access to health services, directly impacting oral health conditions. In addition, information bias cannot be ruled out. The service involved a team composed of different professionals, even using a standardized collection instrument.
Statistical analysis was performed using SPSS version 13 (IBM, Armonk, New York, USA). The descriptive analysis included continuous variables presented as measures of central tendency and dispersion (mean ± standard deviation, or median and variation), and categorical variables presented as absolute and relative values. Despite being a follow-up study, analysis techniques from traditional cohort studies were not used and there was no loss to follow-up. To assess the association between oral candidiasis and RA, a generalized estimation equation model was used, which considered fixed effects for repeated measures. This model has some advantages in longitudinal studies, such as when there is a loss of information from any individual in the sample. In addition, it is possible to include all individuals regardless of the duration of follow-up.
Data adjustment was performed using the Kolmogorov-Smirnov adjustment. Owing to the nonparametric distribution of data, the outcome variable (oral candidiasis) was normalized by taking the Neperian (natural) logarithm. A few interaction terms were also included in an attempt to search for other associations. In order to evaluate the effect-modifying variables, interaction models were performed between them, the predictive factors and their association with the occurrence of oral candidiasis individually.
This research was submitted for analysis by the Research Ethics Committee of the University of Cuiabá, and was approved under protocol 2.227.346 and each participant signed an informed consent form.
3. RESULTS
The present study evaluated 39 patients, followed between January 2018 and December 2019, by a total of 76 evaluations during this cohort study; 32 patients (82.0%) were female and 7 patients (17.9%) were male. The age of patients at diagnosis ranged from 18 to 87 years, with an average of 53.2 (± 6,2) years. Regarding skin color, 20 patients (51.2%) were black, 11 patients (28.2%) were brown, 7 patients (17.9%) were white, and 1 patient (2.5%) was yellow. As for the municipality of residence, 20.5% of the patients were from Cuiabá and the remaining patients were from the interior of the state of Mato Grosso. The most frequently achieved level of education was “completed high school,” with 16 patients (41.0%). The distribution of sociodemographic variables is shown in Table 1.
| Variable | N | % |
|---|---|---|
| Sex | ||
| Female | 32 | 82.0 |
| Male | 7 | 17.9 |
| Age group | - | - |
| 18–38 years | 5 | 12.8 |
| 39–59 years | 22 | 56.4 |
| ≥ 60 years | 12 | 30.7 |
| Color of skin | - | - |
| Brown | 11 | 28.2 |
| Black | 20 | 51.2 |
| White | 7 | 17.9 |
| Yellow | 1 | 2.56 |
| Education | - | - |
| Illiterate | 1 | 2.56 |
| Incomplete elementary school | 10 | 25.6 |
| Complete elementary school | 5 | 12.8 |
| Incomplete high school | 6 | 15.3 |
| Complete high school | 16 | 41.0 |
| Incomplete higher education | 1 | 2.56 |
| Complete higher education | 0 | --- |
Regarding the drugs used to treat RA, methotrexate was the most commonly administered drug to 32 patients (82.0%), followed by prednisone to 25 patients (64.1%), and hydroxychloroquine to 17 patients (43.5%). Among the biological medicines, adalimumab and tocilizumab were each administered to 8 patients (20.5%) (Table 2).
| Variable | N | % |
|---|---|---|
| Steroidal anti-inflammatory | ||
| Prednisone | 25 | 64.1 |
| Synthetic immunosuppressants | ||
| Methotrexate | 32 | 82.0 |
| Leflunomide | 4 | 10.2 |
| Hydroxychloroquine | 17 | 43.5 |
| Biological immunosuppressants | ||
| Abatacept | 3 | 7.7 |
| Adalimumab | 8 | 20.5 |
| Tocilizumab | 8 | 20.5 |
During the follow-up period, 39 patients were evaluated and 12 had oral candidiasis (30.8%). Of the 76 follow-up visits, oral candidiasis was diagnosed 20 times (26,3%). In these cases, 5 (25.0%) had clinical oral mucosal manifestations suggestive of candidiasis, where erythematous type was present in 4 cases and pseudomembranous type was present in 1 individual (Fig. 1). All of them were confirmed by cytopathological examination. In 15 (75.0%) participants, manifestations in the oral mucosa indicative of candidiasis were not observed; however, the cytopathological examination was diagnostic of candidiasis. These cases were then considered as sub-clinical candidiasis, where cytopathological features corresponding to Candida spp. infection was present, but the lesion had not yet been identified clinically (Table 3 and Fig. 1).
| Variable | Times | % |
|---|---|---|
| Candidiasis | - | - |
| Presence | 20 | 26.0 |
| Absence | 56 | 74.0 |
| Hyposalivation | ||
| Presence | 23 | 72.0 |
| Absence | 9 | 28.0 |
When analyzing the variables associated with oral candidiasis, in the crude (univariate) analysis, the use of prednisone (odds ratio (OR)=8.3, 95% confidence interval (CI) 1.72–40.2) and tocilizumab (OR=3.75, 95% CI 1.11–12.5) were predictive factors for the outcome variable (Table 4).
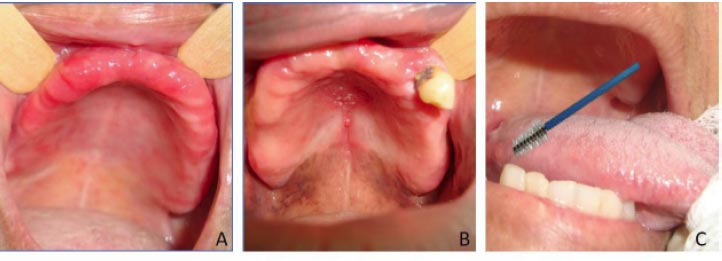 |
Fig. (1). ( A) Participants with clinical oral candidiasis. ( B) Participants with normal oral mucosa and smears were collected only from the bilateral borders of the tongue. |
| Variable | OR | CI | OR Adjusted | CI | P-value | |
|---|---|---|---|---|---|---|
| Male sex | 1.17 | (0.31–4.33) | 9.90 | (0.91–1.05) | 0.10 | |
| Prednisone | 8.33 | (1.72–40.2) | 11.5 | (1.50–88.9) | 0.01 | |
| Tocilizumab | 3.75 | (1.11–12.5) | 5.51 | (1.29–23.5) | 0.02 | |
| Methotrexate | 1.57 | (0.15–16.3) | - | - | - | |
| Leflunomide | 9.10 | (0.17–24.8) | - | - | - | |
| Chloroquine | 1.22 | (0.33–4.54) | - | - | - | |
| Abatacept | 4.66 | (0.60–36.1) | - | - | - | |
| Adalimumab | 1.50 | (0.31–7.25) | - | - | - | |
| Hyposalivation | 2.32 | (0.63–8.78) | - | - | - | |
| Abatacept: male sex | 3.58 | (0.36–33.3) | 7.33 | (0.93–2.86) | 0.08 | |
| Hyposalivation: male sex | 1.68 | (0.46–6.13) | 1.80 | (1.01–3.39) | < 0.001 | |
In the analysis using the multivariate generalized estimation equation, the use of prednisone (OR=11.5, 95% CI 1.50–88.9) and the presence of hyposalivation during the study in male patients (OR=1.80, CI 1.01–3.39) remained associated with the occurrence of candidiasis. Although the use of the biological medicine abatacept by male individuals increased the occurrence of candidiasis by 7.3 times, the association was not statistically significant (p=0.08).
The other drugs for the treatment of RA—methotrexate, leflunomide, chloroquine and adalimumab were not significantly related to the occurrence of oral candidiasis, nor was hyposalivation, when analyzed individually.
4. DISCUSSION
This is the first study to be published that evaluates predictive factors for oral candidiasis in an exclusive cohort of RA patients. Of the 39 patients evaluated, 12 had oral candidiasis (30.8%). Of the 76 follow-up visits, oral candidiasis was diagnosed 20 times (26%), and only 10% of patients with oral candidiasis experienced recurrence over the study period, reflecting the ability of stomatological follow-up within this group of patients to reduce infection occurrence. Cytopathological examination was an efficient diagnostic tool and was accepted well by the participants. Besides confirming the suspected cases of candidiasis, this method also identified the disease in cases in which no signs of candidiasis were identified, and we do recommend it as part of the protocol for these patients [ 17]
Bivariate analysis revealed a statistically significant association between the use of both prednisone and tocilizumab and oral candidiasis in the evaluated patients. In the multivariate analysis, the generalized estimation equation model demonstrated that, over time, the use of prednisone and the presence of hyposalivation in male patients increased the chance of developing oral candidiasis.
The present study demonstrated, via both bivariate and multivariate analyses, that prednisone increased the chance of developing oral candidiasis in patients who used this medication. This medication is a synthetic steroidal anti-inflammatory agent with potent anti-inflammatory action and is widely used for the symptomatic treatment of RA. It affects the functioning of polymorphonuclear cells, macrophages, and T cells, generating an intense and immediate effect on joint inflammation. However, in the medium term, it facilitates the proliferation of opportunistic agents [ 18-20]. As demonstrated in previous studies, the use of corticosteroids and other immunosuppressive drugs is associated with a higher occurrence of severe infections. A recent study from China evaluating 269 patients with rheumatic diseases, including RA, also found a greater number of cases of oral candidiasis in patients using oral prednisone, thus demonstrating that glucocorticoid and immunosuppressive therapy can increase susceptibility to fungal infections in such patients [ 21].
The use of tocilizumab also increased the risk of developing oral candidiasis in this study. This medicine is a humanized monoclonal antibody directed against the interleukin 6 (IL-6) receptor that is effective for the treatment of severe, active, and progressive RA in adults, and is used in combination with methotrexate or other DMDs [ 22, 23]. The rate of infection as a serious adverse effect is low, with fungal infections being the most cited [ 24], but they increase slightly in frequency when tocilizumab is administered in combination with methotrexate or DMDs. Vallabhaneni and Chiller [ 25] in a review article, claim that the use of tocilizumab in large doses promotes the manifestation of other invasive fungal infections, in addition to the occurrence of major episodes of candidiasis.
According to the findings of Bishu et al. [ 10] even though C. albicans infections in the oral mucosa are not commonly reported side effects associated with RA, current data suggest that biological drugs targeted selectively to the IL-23/IL-17 axis may increase the risk of candidiasis in the oral mucosa of RA patients. The T helper 17 (Th17)/IL-17 axis is vital for immunity against fungi, especially the commensal fungus C. albicans. Individuals with RA exhibit impairments already identified in the oral immune response to this fungus, and this impaired response is associated with an increased rate of oral colonization by C. albicans and reduced production of IL-17A-dependent antimicrobial peptides in saliva [ 10].
Numerous oral conditions in patients with connective tissue diseases can result from hyposalivation, particularly oral candidiasis [ 26-31]. In the present study, hyposalivation was observed in 72% of clinical patient evaluations and increased the risk of developing oral candidiasis in male patients. No studies have previously documented this specific association with hyposalivation in males. However, this finding highlights the fact that male patients could anticipate these side effects and seek to minimize hyposalivation through the use of salivary substitutes or other therapeutic approaches. Components of saliva that are important for fungicidal action are histatin 5, which has a fungicidal capacity, and abundant mucin in unstimulated saliva, which provides oral defense through the selective control of fungal adhesion and the ability to kill directly via the formation of intracellular pores [ 18]. The association between oral candidiasis and hyposalivation has already been demonstrated in patients with rheumatic diseases, including RA, where the salivary flow rate in a group with oral candidiasis was significantly lower than that in a control group [ 21, 30].
Patients with RA may also develop secondary Sjögren's syndrome and thus increase the risk of oral candidiasis [ 9]. In Sjogren’s syndrome, Billings et al. [ 18] demonstrated that hyposalivation, identified in both unstimulated and stimulated salivary flow, was significantly associated with oral candidiasis. However, the association was greater when unstimulated flow was evaluated. In the present study, we decided to evaluate total unstimulated saliva, since it reflects the flow at rest, which is responsible for lining the oral mucosa and maintaining oral tissue, whereas stimulated saliva is secreted only in response to a stimulus and reflects the functional flow of the gland [ 18].
4.1. Limitations of the Study
The possible limitations of the present study deserve some comments. Although the risk factors for oral candidiasis available were adjusted, the results may still have been affected by other factors that were not evaluated or unknown, such as, for example, the oral health status of the patients, the dosage and association of the drugs and even the clinical features of oral candidiasis. Another consideration is that the medical records were not digitized and the care was not carried out by a single professional but involved a team that today makes up the oral diagnosis clinic, where many times there was no filling in with all the necessary information even with a medical record. In addition, patients did not always return for scheduled re-appointments, thus impairing the follow-up of part of the treatment cohort participants, in addition to being possible to include all individuals regardless of the follow-up time of these patients. Another advantage of this statistical analysis technique is the possibility of analyzing samples with smaller sizes and still being able to demonstrate statistically significant associations between the explanatory variables and the dependent variable [ 32]. As for external validity, this study was carried out in patients who are under outpatient follow-up at the hospital level of a public health institution. A limitation is the generalization of these results in other institutions, such as private institutions.
4.2. Results
The results of the present study emphasize the importance of stomatological evaluation and frequent monitoring of patients with RA, even before starting treatment for the disease, given that they are at a higher risk of infection. Oral candidiasis is the most common fungal infection of the oral mucosa, and its predictive factors are well-studied. However, the analysis of patients with rheumatic diseases is still incipient and requires further study, especially in the case of RA, which is a chronic disease requiring immunomodulatory drugs throughout the life of the affected individual.
CONCLUSION
In conclusion, this study demonstrated that prednisone and tocilizumab administration, and hyposalivation in male patients are risk factors for oral candidiasis in patients with RA, thus reinforcing the importance of stomatological monitoring, a preventive approach, and early diagnosis of this pathology.
LIST OF ABBREVIATIONS
| RA | = Rheumatoid Arthritis |
| DMDs | = Disease-modifying Drugs |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research was approved by submitted to analysis by the Research Ethics Committee of the University of Cuiabá and was approved under protocol 2.227.346.
HUMAN AND ANIMAL RIGHTS
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or research committees and with the 1975 Declaration of Helsinki, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was obtained from all participants of this study.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
STANDARDS OF REPORTING
STROBE guidelines were followed.
FUNDING
This study was supported by the Foundation for Research Support of the State of Mato Grosso FAPEMAT (EDITAL Nº 003/2017 – PPSUS – PROCESSO No. 334993/2018)
CONFLICT OF INTEREST
The authors declare no conflict of interest financial or otherwise.
ACKNOWLEDGEMENTS
We thank Ph.D. Luiz Sergio Guedes Barbosa and Christina Paesano Marques Garcia for their technical help.







