Hepatitis B Serology in Patients with Rheumatic Diseases
Abstract
Background:
Only limited data are available on the prevalence of hepatitis B in patients with proven rheumatic diseases and thus the risk of reactivation under immunosuppressive therapy.
Objective:
To analyse hepatitis B serology in patients with rheumatic diseases prior to therapy.
Method:
In total, 1,338 patient records were analysed for HBsAg, HBsAb and HBcAb in a cross-sectional, single-centre study between 2011 and 2015 at first presentation. Data acquisition was realized using electronic patient files created during routine care. The main variables considered as predictors for HBV reactivation included (i) the exact type of rheumatic disease and (ii) the therapeutically induced immunosuppression.
Results:
Overall, 5.9% of patients (n=79) had proven contact with hepatitis B (HBcAb positive), and HBsAb were not detected in 1.3% (n=18). The rate of vaccinated subjects was 7.8%. HBsAg was detected in 3 patients (0.2%). In addition, 70.3% of patients were treated during the course of rheumatologic disease previously or currently with glucocorticoids, 85.2% with disease-modifying anti-rheumatic drugs (DMARDs) and 20.1% with a biologic agent (e.g., anti-IL-6, anti-TNFalpha, anti-CD20, CTLA4Ig or anti-IL-12/23).
Conclusion:
Prevalence of hepatitis B serostatus in the analysed rheumatic patients regarding HBs-Ag and HBcAb with or without HBsAb prior to therapy does not differ from the data published for the general population in Germany. However, the rate of hepatitis B vaccinated patients was lower. In general, a significant portion of patients (5.9%) has been exposed to HBV and therefore exhibited an increased risk of reactivation of hepatitis B when undergoing immunosuppressive therapy.
INTRODUCTION
Only limited data are available on the prevalence of viral hepatitis B (HBV) in patients with proven rheumatic diseases. Given that immunosuppressive therapy can lead to a reactivation of hepatitis B virus (HBV) [1-3], the Center for Disease Control and Prevention (CDC) recommends screening patients for hepatitis B surface antigen (HBsAg), hepatitis B core antibody (HBcAb), and hepatitis B surface antibody (HBsAb) before starting immunosuppressive therapy [4]. Additionally, the American Association for the Study of Liver Diseases (AASLD) and the 2008 National Institutes of Health Consensus Development Conference on Hepatitis B recommend HBV screening before initiating immunosuppressive therapy [5, 6]. In the field of rheumatic diseases, guidelines for the treatment of rheumatoid arthritis by the American College of Rheumatology (ACR) or the European League against Rheumatism (EULAR) also suggest screening, include recommendations on the management of HBV and even endorse the use prophylactic antiviral therapy during immunosuppressive therapy in some cases, particularly if B-cell depleting therapy is applied [7, 8]. However, minimal information is known about the prevalence of hepatitis B infection in the rheumatologic setting, although most of these patients receive immunosuppressive therapy for many years and in some cases even up to a lifetime with variable degrees of risk for reactivation of HBV during the course of disease [9].
The reactivation of hepatitis B infection under immunosuppressive treatment entails considerable risk for the patient. In addition to elevated transaminase counts and clinical signs of hepatitis, patients may suffer serious complications, such as liver failure, liver cirrhosis or hepatocellular carcinoma (HCC); the outcome may even be fatal. Hepatitis B virus reactivation has been best studied in patients receiving chemotherapy for haematologic or solid neoplastic disorders [10-12]. However, reactivation can also occur in patients receiving antirejection treatment after stem cell or organ transplantation, long-term corticosteroid therapy, and B-cell depleting Anti-CD20 therapy or other intensive immunosuppressive therapies in a rheumatologic setting. Most cases of HBV reactivation occur in patients who are hepatitis B surface antigen (HBsAg) positive, but reactivation has also been reported in patients who are HBsAg negative but positive for the hepatitis B core antibody (HBcAb). Furthermore, repetitive treatment with B lymphocyte depleting agents can lead to a decrease in protective HBsAb and thereby increase the risk of reactivation [13, 14].
The primary objective of this study was to evaluate the distribution of hepatitis B serologic markers in a large rheumatic cohort in Germany prior starting therapy. We intended to provide a first impression regarding the percentage/portion of patients at risk for reactivation of hepatitis B under immunosuppressive treatment. Therefore, hepatitis B serology was analysed in our database; the analysis included 1,338 patients with available hepatitis B serology.
MATERIALS AND METHODOLOGY
This report utilizes data from a single-centre, cross-sectional study that included patients (n=1,338) with proven rheumatic disease, e.g., rheumatoid arthritis, axial and peripheral predominant spondyloarthritis, connective tissue diseases, vasculitis, and other inflammatory disorders (e.g., polymyalgia rheumatica, gouty arthritis, autoinflammatory disorders) in the rheumatologic context.
The basic variables assessed included underlying rheumatic disease and serology for hepatitis B (HBsAg, HBcAb, and HBsAb) at the first presentation of the patient. Antibodies against HBs and HBc were analysed by electro-chemiluminescence immunoassay (ECLIA), and HBsAg was analysed by chemiluminescence immunoassay (CLIA). Patient records created between 2011 and 2015 were analysed. Data were assessed during routine care according to available recommendations and guidelines. No further inclusion or exclusion criteria were applied.
Data management and statistical analyses were performed for all data as appropriate using Microsoft Excel [15] or SPSS software [16], respectively. All performed inferential tests were considered to be statistically significant at P<0.05. Pearson chi-square tests were used to compare frequencies of categorical variables between patient subgroups. Moreover, analysis of variance (one-way ANOVA) was performed to test for mean differences in continuous variables between independent patient subgroups. Post-hoc analyses were utilized where appropriate (Scheffé test for ANOVA models, standardized/z-transformed residuals for chi-square tests).
RESULTS
The cohort investigated included 1,338 electronic records of patients with proven rheumatic disease. Hepatitis B serology was available for all patients. The collective included 580 (43.3%) male and 758 (56.7%) female patients. The average age was 60.98 years. In addition, 58 patients (4.3%) predominantly suffered from axial spondyloarthritis, 226 patients (16.9%) predominantly suffered from peripheral spondyloarthritis (e.g., psoriatic arthritis), 36 patients (2.7%) suffered from vasculitis, 187 patients (14.0%) suffered from other inflammatory disorders (e.g., gouty arthritis, polymyalgia rheumatica) and 65 patients (4.9%) suffered from connective tissue diseases. Moreover, 766 patients (57.2%), the largest patient group, suffered from rheumatoid arthritis (Table 1).
| n=1,338 | |
|---|---|
| Sex | |
| female | 758 (56.7%) |
| male | 580 (43.3%) |
| Age [years] | 60.98 |
| Rheumatologic diagnosis | |
| Axial spondyloarthritis | 58 (4.3%) |
| Other inflammatory disorders | 187 (14.0%) |
| Connective tissue disease | 65 (4.9%) |
| Peripheral spondyloarthritis | 226 (16.9%) |
| Rheumatoid Arthritis | 766 (57.2%) |
| Vasculitis | 36 (2.7%) |
| HBV serostatus | |
| HBsAg [-], HBsAb [-], HBcAb [-] | 1,154 (86.2%) |
| HBsAg [-], HBsAb [+], HBcAb [+] | 58 (4.3%) |
| HBsAg [-], HBsAb [-], HBcAb [+] | 18 (1.3%) |
| HBsAg [-], HBsAb [+], HBcAb [-] | 105 (7.8%) |
| HBsAg [+] | 3 (0.2%) |
The majority of patients (n=1,154; 86.2%) did not have contact with hepatitis B and were not vaccinated against HBV. HBcAb was identified in 76 of the patients (5.6%), which is consistent with actual contact with hepatitis B. HBsAb were simultaneously identified in 58 of the patients (4.3%), whereas 18 patients (1.3%) only exhibited HBcAb. HBsAg was noted in 3 patients (0.2%) with positivity for HBV DNA in two out of these patients. Only HBsAb were detected in 105 patients (7.8%), reflecting a vaccination status (Table 1). An age-based sub-group post-hoc analysis was performed for the group of vaccinated patients. The group of patients less than 30 years of age demonstrated a significantly higher vaccination rate (41.7%) compared with the group of patients older than 30 years of age (6.3%) (P<0.001).
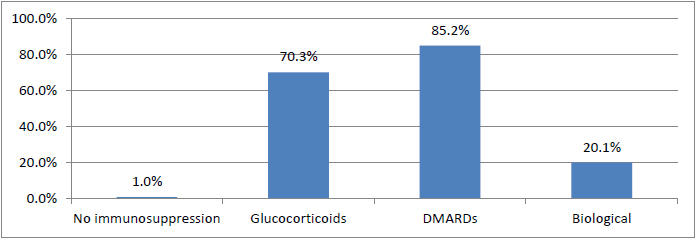
Immunosuppressive therapy was grouped by use of glucocorticoids, conventional DMARDs or biological DMARDs at some point in the course of disease. Glucocorticoids were used in the course of the disease in 70.3% of cases, and 85.2% were treated with conventional disease modifying anti rheumatic drugs (DMARDs), such as methotrexate, leflunomide, hydroxychloroquine, sulfasalazine, cyclosporine or azathioprine. A total of 269 patients (20.1%) were treated using a biologic agent at some point (e.g., anti-IL-6, anti-TNFalpha, anti-CD20, CTLA4Ig or anti-IL-12/23). The group of patients treated with a biologic agent included a total of 33 patients treated with rituximab (Fig. 1).
An analysis of the individual diagnosis categories (Table 2) revealed a significantly higher rate of those vaccinated in the axial spondyloarthritis group (19.0%, P<0.001). However, the other findings with regard to serostatus did not differ on the basis of the underlying rheumatologic diagnosis.
| HBsAg [-] HBsAb [+] HBcAb [-] |
HBsAg [-] HBsAb [-] HBcAb [+] |
HBsAg [-] HBsAb [+] HBcAb [+] |
HBsAg [+] HBsAb [-] HBcAb [+] |
HBsAg [-] HBsAb [-] HBcAb [-] |
|
|---|---|---|---|---|---|
| Axial spondyloarthritis | 11 (19.0%)* | 2 (3.4%) | 2 (3.4%) | 0 (.0%) | 43 (74.1%) |
| Other inflammatory disorders | 7 (3.7%) | 2 (1.1%) | 5 (2.7%) | 0 (.0%) | 173 (92.5%) |
| Connective tissue disease | 5 (7.7%) | 0 (.0%) | 2 (3.1%) | 0 (.0%) | 58 (89.2%) |
| Peripheral spondyloarthritis | 34 (15.0%) | 2 (.9%) | 10 (4.4%) | 0 (.0%) | 180 (79.6%) |
| Rheumatoid Arthritis | 45 (5.9%) | 11 (1.4%) | 39 (5.1%) | 3 (.2%) | 668 (87.2%) |
| Vasculitis | 3 (8.3%) | 1 (2.8%) | 0 (.0%) | 0 (.0%) | 32 (88.9%) |
At 13.5%, the percentage of vaccinated individuals in the group of patients treated with biologics was also significantly (P<0.001) increased compared with the group of patients without biologic therapy in their treatment history (data not shown). The other findings with regard to serostatus did not differ on the basis of the therapy applied during course of disease. In a post-hoc analysis, the significantly increased vaccination rate could be attributed to the lower total average age of the patients both in the group of axial spondyloarthritis and the biologics cohort. The serostatus of patients who underwent rituximab treatment within the biologics patients was investigated in another sub-group analysis. A risk constellation for reactivation was noted in only two out of 33 patients (HBcAb [+]/HBsAb [-] and HBcAb [+]/HBsAb [+], respectively).
The analysis of ASAT, ALAT and GammaGT revealed no significant differences based on hepatitis B serostatus. In the group of patients positive for HBcAb, no difference was noted in relation to the other serogroups.
Prior to starting therapy, three patients (0.2%) were diagnosed positive for HBsAg and HBcAb (HBsAb negative) and rheumatoid arthritis for the first time. Two patients out of these were also positive for HBV-DNA (data not shown). One of these patients, a 77-year-old female patient, also required immunosuppressive treatment for late-onset rheumatoid arthritis (LORA). Parallel to DMARD therapy consisting of a low dosage of prednisolone (initially 10 mg prednisolone) and leflunomide (MTX contraindicated due to renal insufficiency), she was treated with an adapted dose of entecavir. The second patient was treated with methotrexate, low dose glucocorticoids and entecavir. HBV DNA levels subsequently decreased below the threshold of detectability in both cases. The third patient with positivity for HBsAg was initially negative for HBV DNA and was monitored at regular intervals for HBV DNA under treatment with methotrexate and low dose glucocorticoids without reactivation during period under review.
A 63-year-old patient with rheumatoid arthritis who initially tested positive for HBcAb but negative for HBsAb suffered from reactivation of hepatitis B during the first few months of DMARD therapy with MTX and prednisolone. After initiating treatment with entecavir and temporarily discontinuing the DMARD treatment, she was able to resume treatment with MTX while continuing to take entecavir and dispensing with glucocorticoids. This caused the HBV DNA levels to decrease below the threshold of detectability (data not shown).
DISCUSSION
In most cases, patients with inflammatory rheumatic diseases receive immunosuppressive treatment, which generally increases the risk of infection. In addition to acquiring new infections, patients may experience a reactivation of dormant infections, e.g., latent tuberculosis, herpes zoster or hepatitis B [17-20]. The natural course of hepatitis B virus infection is determined through the interaction between viral replication and the host's immune competence. HBV persists in all patients with infection, even those with evidence of serological recovery. Thus, individuals with a history of HBV infection who receive immunosuppressive therapy are still at risk for reactivation (usually defined by an increase in HBV DNA). In general the risk for reactivation seems to be lower in the autoimmune setting even in patients with HBcAb compared to immunosuppressive treatment in haematological or oncological disorders [21-25]. The very low rate of HBV reactivation in our cohort may also suggest that immunosuppressive therapy in the rheumatologic setting seems to be safe. Nevertheless, we did not perform a prospective analysis of the actual reactivation rate by serological monitoring during therapy unless it was recommended by guidelines. The primary goal of our study was to analyse hepatitis B serology in patients with rheumatic diseases prior to therapy as the patient’s serologic status, as well as the type of applied immunosuppressive therapy determine risk of reactivation [1, 2, 26-30]. The EASL (European Association for the Study of Liver) guidelines also point out to the risk of reactivation in HBsAg positive patients receiving chemotherapy or immunosuppressive therapy, particularly if rituximab is given alone or in combination with steroids. Therefore, screening for HBsAg and HBcAb prior to chemotherapy and immunosuppressive therapy is strongly recommended by the EASL. HBsAg positive patients should be tested for HBV DNA and receive antiviral therapy during immunosuppression (regardless of HBV DNA levels). HBsAg negative patients with positive HBcAb are supposed be tested for HBV DNA. HBcAb positive patients with detectable serum HBV DNA are recommended to be treated, while HBcAb positive patients without detectable serum HBV DNA who receive chemotherapy and/or immunosuppression should be followed carefully testing for ALAT elevation and HBV DNA [31]. The American Gastroenterological Association (AGA) and the American Association for the Study of Liver Diseases (AASLD) also recommend the screening of patients who will be receiving cancer chemotherapy or immunosuppressive therapy [1, 32, 33]. Nevertheless, there seem to be some controversies among different guidelines regarding prophylaxis of HBV reactivation in immunosuppressed patients [34].
The available rheumatology guidelines recommend screening for latent tuberculosis and hepatitis B and C, particularly before starting biologicals. However, no standardized, consensus-based process is currently available for screening patients for viral hepatitis if they are to be treated with glucocorticoids or conventional disease-modifying anti rheumatic drugs (DMARDs), such as methotrexate, leflunomide or azathioprine [35, 36], and this class of patients comprises the vast majority of patients. In addition, only limited data are available on the prevalence of viral hepatitis B in patients with proven rheumatic diseases at all. For the first time in a collective of this size, the current study assesses the serostatus of patients with a proven diagnosis of inflammatory rheumatic disease in a routine clinical setting, covering all observed indications prior to therapy. The distribution of the diseases in our cohort and the treatment groups corresponds with pre-existing data on the rheumatologic patient population in Germany [37]. The percentage of patients in our cohort who were previously treated with a biological agent was 20.1%, and this value is compatible with the published biological prescription rate in Germany [38].
HBcAb was identified in 5.6% of patients in our cohort, indicating the percentage of individuals who had contact with wild type HBV in the past. In a study of 7,047 subjects conducted by the Robert Koch Institute (RKI) in Germany between 2008 and 2011 [39] 5.1% of adult subjects tested positive for antibodies against the core antigen (HBcAb). The high concordance in observed HBcAb rates indicates that our cohort is representative. However, in the RKI study, only 0.6% of 18 to 79 year olds in Germany tested positive for HBcAb exclusively, i.e., half the percentage found in our collective (1.3% of subjects), indicating that these patients were at particular risk of reactivation when undergoing immunosuppressive treatment. The rate of HBcAb exclusive positivity was greater than two-fold increased as the percentage of the general German population, but the low absolute number in our study sample (n=18) must be taken into account. Nevertheless, the increased percentage of HBcAb carriers is particularly surprising and must be confirmed in further studies. The prevalence of samples that tested positive for HBcAb and HBsAb (indicating recovery from hepatitis B infection) was 4.1% for Germany compared with 4.3% in our cohort. Neither the RKI study nor our data showed any gender-related discrepancies. Interestingly, a multivariate logistic regression model used to analyse the RKI data for the general population adjusted for age and gender revealed an association between hepatitis B infection and socioeconomic status (SES) [low vs. high SES: OR 3.82 (2.57-5.66); middle vs. higher SES: OR 1.76 (1.20-2.58)]. In other words, the lower the individual’s socioeconomic status, the higher the risk of hepatitis B infection. The RKI tested 22.9% of 18- to 79-year-old men and women in Germany exclusively positive for HBsAb, indicating immunity against hepatitis B as the result of vaccination. Compared with an older German study dating from 1998, the prevalence of isolated HBsAb positivity during the period from 2008 to 2011 was significantly increased, indicating wider vaccination coverage. This development may be largely attributed to the changes in vaccination recommendations. Vaccinations have been recommended for groups with an increased risk of infection since 1982 (West Germany) and 1984 (East Germany). In 1995, the Standing Committee on Vaccination (STIKO) issued a general recommendation for vaccinating newborns against HBV and administering late vaccinations to older children and young people with no immunity. The rate of people vaccinated against hepatitis B in our cohort was only 7.8% overall. The increased average age of 60.98 years compared with the general population in Germany in the RKI study likely explains the low rate because we found that the percentage of subjects vaccinated varied between age groups and was significantly increased in patients younger than 30 years (41.7% versus 6.3%, P<0.001). In concordance, the higher rate of vaccinated individuals in the axial spondyloarthritis patient group and the group of patients with a biologic therapy also achieved statistical significance (P<0.001 and P<0.004, respectively) due to a lower average age found for both groups in the post-hoc analysis. The lower average age of patients on the initial manifestation of axial spondyloarthritis compared with rheumatoid arthritis, for example, is not a novel finding, but the patients treated using a biologic were also younger on average; thus, age would explain the increased rate of vaccinated patients in both cases. Nevertheless, the strikingly low rate of vaccinated individuals in the group of patients treated with a biologic was surprising, especially given that the Standing Committee on Vaccination (STIKO) clearly recommends hepatitis B vaccination as an indication-based vaccine for people in Germany who are expected to experience a severe progression of hepatitis B disease on account of pre-existing or anticipated immunodeficiency or -suppression. Ultimately, the low rate of vaccination against hepatitis B is therefore perfectly reflective of the fundamentally and obviously limited implementation of official recommendations for the collective of patients undergoing immunosuppressive treatment [40].
HBsAg was detectable in three patients (0.2%), which is almost identical to the results of the Robert Koch Institute’s study (0.3%). Data from the USA revealed HBcAb carrier status in 4.7% of subjects and chronic hepatitis B in 0.27% for the period from 1999 to 2006 [41], which closely mirrors the results obtained in Germany by the RKI for the period from 2008 to 2011. The percentage of HBcAb carriers among the younger population of the USA was again lower than that during the observation period from 1988 to 1994, as the percentage of people vaccinated was higher (56.7% in the 6-19 age group). European Centre for Disease Prevention and Control data for Europe and the Near East reveal various significant regional discrepancies with regard to viral hepatitis: >5-10% of subjects in Albania tested positive for HBsAg and antiHCV compared with the above-mentioned 0.3% in Germany who tested positive for hepatitis B or C [42]. In total, 79 patients (5.9%) in our cohort have been exposed to HBV (58 patients HBsAg [-]/HbcAb [+]/HBsAb [+], 18 patients HBsAg [-]/HbcAb [+]/HBsAb [-], and three patients HBsAg [+]). Our data for patients with proven rheumatic diseases thus largely conforms to the data obtained by the RKI for Germany. Unfortunately, we have no data on the subjects’ countries of origin or migrational status. In addition, their socioeconomic status was not assessed. However, it seems probable that there should be no significant difference in distribution between the rheumatology patients tested and the general population.
Elevated transaminases are a common manifestation in rheumatology patients during the disease course and must be clarified not only with regard to toxic, drug-related causes, concomitant diseases or the underlying rheumatic disease but also to a reactivated or hitherto undetected viral hepatitis [43]. The analysis of ASAT, ALAT and the GammaGT in our cohort revealed no significant differences based on the hepatitis B serostatus. Thus, if the infection risk and/or the risk of reactivation are to be estimated, there is an absolute need to analyse the hepatitis B serostatus.
No difference regarding HBcAb was noted in our collective based on the underlying rheumatologic disease or the need to escalate treatment and administer biologicals. Instead, the results were spread evenly between the rheumatological diagnoses. Similar to our data, the literature does not indicate any association with inflammatory rheumatic diseases except for cryoglobulinaemic (secondary) vasculitis, which is classified as a direct extrahepatic manifestation of chronic hepatitis C, or the secondary polyarteriitis nodosa associated with hepatitis B [44, 45]. Moreover, the literature does not contain any observation that inflammatory rheumatic disease may progress more aggressively depending on the patient’s hepatitis B serostatus [46].
Only little data are available on the seroprevalence of hepatitis B in rheumatologic patients, but even less reliable prospective data on the risk of reactivation under continued immunosuppression are available [47-49]. Although anticytokine-based biologicals (e.g., anti-TNFalpha) appear to be associated with a relatively low risk of hepatitis B reactivation [50], the rate of HBV reactivation after treatment with B cell depleting rituximab (Anti-CD20) has increased significantly in recent years. This complication is occasionally fatal [51-53]. The serostatus of patients who underwent rituximab treatment within the biologics patients in our cohort was investigated in another sub-group analysis. A risk constellation for reactivation was noted in only two out of 33 patients (HBcAb [+]/HBsAb [-] and HBcAb [+]/HBsAb [+], respectively). The patients in question are undergoing assessment for HBsAg and HBV DNA at regular intervals. Furthermore, immunization against hepatitis B is planned in the interval before repeating the next rituximab dose for rheumatoid factor and ACPA-positive (anti-citrullinated peptide antibody) rheumatoid arthritis in the patient with HBcAb [+]/HBsAb [-]. Based on current recommendations, a prophylactic anti-viral treatment with a nucleos(t)ide analogue is not necessarily advisable in this situation. The current S3 guidelines for the diagnosis and treatment of hepatitis B in Germany recommend treating HBsAg-positive or HBV DNA-positive patients (occult hepatitis B) antivirally with a nucleos(t)ide analogue in case of immunosuppressive treatments. HBsAg-negative and HBcAb-positive patients on the other hand should be monitored closely, and anti-viral treatment is only indicated in the event of an increase in HBV DNA or evidence of HBsAg [3].
Our cohort contained only one proven case of hepatitis B reactivation (interestingly, under treatment with a conventional synthetic DMARD and glucocorticoids as described above). In this context, the authors believe that the use of glucocorticoids and DMARDs as well as the use of biologicals (in our cohort the majority of patients was treated with glucocorticoids at some time) must be addressed. The influence of glucocorticoids on the risk of infection has been revealed in recent years [54, 55]. Depending on the dose administered and the length of treatment that rheumatological patients are required to undergo, glucocorticoids significantly increase the risk of infection, even compared with biologicals [56]. However, it is not possible to dispense with glucocorticoids completely, and these agents may be particularly necessary during the early phases of inflammatory rheumatic disease. In the authors’ view, the patient’s hepatitis B serostatus should therefore be established at the beginning of immunosuppressive treatment, particularly if moderately high (>20 mg prednisolone equivalent) or high doses of glucocorticoids are to be administered.
There is obviously a lack of data on the actual reactivation rate under continuing immunosuppression from larger collectives of patients, e.g., those undergoing long-term treatment with glucocorticoids or conventional DMARDs, who comprise the largest collective. Our data on the HBV serostatus prior to therapy therefore may increase awareness of this issue. Current guidelines already recommend screening for HBsAg and HBcAb prior to immunosuppressive therapy. This is particularly important in view of the significant regional discrepancies revealed by the ECDC’s data on Europe (described above) and the increasing proportion of people with migrant backgrounds in Western industrial countries. There is still a need for prospective data facilitating better calculation of the risk of viral hepatitis reactivation depending on the patient’s serostatus and the risk factors at play.
CONCLUSION
Prevalence of hepatitis B serology in the analysed rheumatic patients regarding HBsAg and HBcAb with or without HBsAb prior to therapy does not differ from the data published for the general population in Germany. However, the rate of hepatitis B vaccinated patients was lower. In general, a significant portion of patients (5.9%) has been exposed to HBV and therefore exhibited an increased risk of reactivation of hepatitis B when undergoing immunosuppressive therapy.
ABBREVIATIONS
| ALAT | = Alanin-Aminotransferase |
| ASAT | = Aspartat-Aminotransferase |
| CTLA4 | = Cytotoxic T-lymphocyte-associated protein 4 |
| DMARD | = Disease modifying antirheumatic drug |
| GammaGT | = Gamma-Glutamyltransferase |
| HBcAb | = Antibody against Hepatitis B core antigen |
| HBsAb | = Antibody against Hepatitis B surface antigen |
| HBsAg | = Hepatitis B surface antigen |
| HBV | = Hepatitis B virus |
| MTX | = Methotrexate |
| TNFalpha | = Tumor necrosis factor alpha |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


