All published articles of this journal are available on ScienceDirect.
Prevalence of Group A Beta-Hemolytic Streptococcus Oropharyngeal Colonization in Children and Therapeutic Regimen Based on Antistreptolysin Levels: Data from a City From Southern Brazil
Abstract
The aim of this study is to determinate the prevalence of oropharyngeal colonization by group A beta-hemolytic Streptococcus (GABHS) in pediatric population of Ponta Grossa, a midsize city of southern Brazil; estimate the effectiveness of antistreptolysin-O (ASO), compared to culture, in presence of infection; and design an unpublished investigative algorithm of rheumatic fever's suspicion, based on needs identified in worldwide consensus. It is an epidemiologic, observational and transversal study, involving 180 children younger than 12 years. Secretion of posterior oropharynx was collected for culture; and peripheral blood for determination of ASO. Student-t and chi-square tests, with Yates correction, were performed for statistical analysis. The ASO cutoff was determined by Receiver Operating Characteristic (ROC) curve. The prevalence encountered was 3.9%, and 25.5% of the children showed reagent ASO. This serological test demonstrated quantitatively and qualitatively significant associations to the GABHS presence (p=0.0001 for both associations) throughout the ROC curve, 200 U Todd was the value that resulted in the best accuracy, demonstrating 100% of sensibility and 80% of specificity in the GAS infection documentation. Also, it was found that the value of 1.200 U represents a specificity of 100%. The results emphasize the need for similar studies in other populations, to provide better targeting of the diagnosis and treatment of oropharyngitis by GABHS, which in turn can prevent up to 80% the cases of rheumatic fever, and consequently, the chronic rheumatic heart disease.
INTRODUCTION
The rheumatic fever (RF), characterized by an autoimmune reaction between antigens from the streptococcal M protein and heart tissue components, with multisystemic features, still presents high rates of incidence and prevalence in developing countries [1-3]. It occurs after successive incidents of exposures to those antigens, through repeated infections of oropharynx by the group A beta-hemolytic Streptococcus [4].
In 2012, 4,731 hospitalizations due to acute rheumatic fever were registered in Brazil [5]. Even with the introduction of antibiotics and recent advances in the knowledge of its pathogenesis, its main and most feared complication, rheumatic carditis (RC) – present in up to 60% of patients [6, 7] remains the leading cause of cardiac surgery in young adults in the country [8].
The diagnosis of RF is essentially clinical, based on major and minor criteria proposed by Jones in 1944. It is necessary to apply two major criteria, or one major and two minor. Among the major criteria are: (a) carditis, in its various forms; (b) migratory polyarticular arthritis; (c) subcutaneous nodules; (d) erythema marginatum and (e) Sydenham's chorea. In contrast, minor criteria include: (i) fever; (ii) prolongation of the electrocardiographic PR space; (iii) arthralgia and (iv) laboratory abnormalities (such as erythrocyte sedimentation rate and elevated c-reactive protein, leukocytosis in blood count) [8-10]. In 1992, the World Health Organization (WHO) began to recommend supplementation with evidence of streptococcal infection. Current modalities include oropharyngeal cultures, antistreptolysin-O (ASO) and anti-DNaseB, although access to the latter is scarce [11]. While oropharyngeal cultures enable the detection of an active infection by GABHS, ASO indicates only one, possibly recent, previous contact with the pathogen [12, 13].
The criteria used in the diagnosis of RF can result in up to 70% false-negative. And in clinical practice, two situations are observed: (1) patients as having been overdiagnosed with RF, based only on the validity of the positive ASO, causing inadvertent use of penicillin, and are even exposed to the risk of bacterial resistance; (2) patients underdiagnosed, usually by over-reliance on the part of the clinical criteria of Jones, which has high rates of false-negative results [14-16]. Thus, the chronic rheumatic heart disease perpetuates itself as a major public health problem [1, 6].
When reviewing the medical literature, even though the sensitivity and specificity of the ASO have been extensively evaluated in the study of rheumatic fever, there were no studies that determined the values of these parameters when correlated to infection of the oropharynx by GABHS, in comparison to culture.
The present study aims to estimate the prevalence of oropharyngeal colonization by the GABHS in the city of Ponta Grossa (PR), as well as to develop an algorithm regarding the presence of this pathogen across the ASO levels, based on the need for consensus pointed in RF [17]. First, however, a brief review of RF will be presented.
MATERIAL AND METHODS
It is an epidemiologic, observational and cross-sectional study, using 180 children, from 0 to 12 years old, attended by Community Health Center Dr. Cleon Francisco de Macedo, located in Jardim Paraíso, a suburb of Ponta Grossa, a medium-sized city in Paraná.
The report was developed in state and municipal schools in the neighborhood, providing the children’s legal guardians with pamphlets explaining rheumatic fever and oropharynx infection caused by group A beta-hemolytic Streptococcus (GABHS). The pamphlets also informed the days in which the collection would occur – acquiring data on oropharynx culture and antistreptolysin-O (ASO), taking place in the community health center. The legal guardian was asked to consent, and the culture collection was only made after the guardian authorized and consented in allowing so. The study was executed in autumn.
In order to obtain the culture of secretions from the posterior oropharynx, next to the tonsils, a sterile swab was used, which was also stored in a sterile Cary-Blair transport medium. For the other exams, 5 ml of blood were collected and separated for serologic determination (ASO).
The material collected by swab was seeded onto plates containing agar blood, using a streaking technique and kept in incubation at 37º C for 48 hours. After that, the microbiologist identified a suspicion of Streptococcus pyogenes in the predominance of beta-hemolytic. The stratification in different groups of Lancefield occurred using latex agglutination test (AVIPATH®Strep), in which the antibody reacts with a group-specific carbohydrate present on the bacteria wall [19]. In case of positive culture for GAE, an antibiogram was used (disk diffusion using Mueller-Hinton agar), whose disk-reading revealed pathogen susceptibility or resistance to penicillin G benzathine and to other antibiotics, such as clindamycin, chloramphenicol, bacitracin, levofloxacin, meropenem, sulphazotrim and vancomycin.
In order to make ASO tests, the tubes were centrifuged (1500 r.p.m, x 5 min). After doing so, the serum was removed. It was determined with latex agglutination test, using AVITEX®ASO kits. Titration “1:1” and “1:5” to all patients, in order to avoid Hook effect (or prozone effect), and subsequent titrations (“1:1,5”; “1:2”; “1:3”, “1:4”, “1:6”) were made, according to its positivity. The result was analyzed after two minutes of circular motion, and it was considered reactive when there was a formation of granules. It was considered as a normal ASO result when inferior to 200 Todd units.
The efficacy of the test in relation to the oropharynx’s culture was applied to the sensitivity and specificity calculation, using electronic calculators. The rate of best accuracy (the one that shows best rates of sensitivity and specificity) was obtained using a Receiver Operating Characteristic (ROC) curve [18]. With this graphical plot, it is possible to stipulate the best rate of sensitivity (vertical axis) and the best rate of specificity (horizontal axis). The statistical significance was determined using a Student’s t-test and chi-squared test with Yates’ correction.
This study was approved by the Research With Human Beings Ethics Committee of the State University of Ponta Grossa, under protocol number 16.148/2011, and authorized by the City Hall and the Municipal Department of Health. All participants’ legal guardians were properly informed and signed a consent.
RESULTS
One hundred and eighty children participated in this study - 87 (48.3%) of them were male and 93 (51.7%) female. The average age was 6.82 years, and the average tends to get higher when analyzing only group A beta-hemolytic Streptococcus carriers. Seven (3.9%) of them showed oropharynx’s culture positive to GABHS, representing 43.75% of all positive cultures for any type of Streptococcus. Other groups were found, such as B, C and G group. The antistreptolysin-O was the reagent in one quarter of the patients (25.5%). The female predominated in positive cultures for any Streptococcus, not-group A and with ASO reagent. The demographic characteristic and initial results can be found in Tables 1 and 2. All strains of GAS were sensible to penicillin G benzathine and other antibiotics that are commonly used in the treatment of its infection.
Basic characteristic of patients and initial results.
| Variable | Patients (n = 180) |
|---|---|
| Age Average (DP) | 6,82 (±2.65) |
| Sex Male Female |
87 (48.3%) 93 (51.7%) |
| Cultures + GAS GBS GCS GGS Total |
7 (3.9%) 3 (1.7%) 4 (2.2%) 2 (1.1%) 16 (8.8%) |
| Reagent ASO | 46 (25.5%) |
Basic characteristic of patients with positive culture and/or ASO reagent.
| Variable | Beta-Hemolytic Streptococcus (n=16) | EGA (n=7) | EGB, EGC e EGG (n=9) | Reagent ASO (n=46) |
|---|---|---|---|---|
| Age Average (DP) | 7.5 (±2.0) | 8.71 (±1.18) | 6.55 (±2.17) | 7.74 (±1.75) |
| Sex Male Female |
5 (31.25%) 11 (68.75%) |
3 (42.86%) 4 (57.14%) |
2 (22.22%) 7 (77.78%) |
21 (45.65%) 25 (54.35%) |
ASO titration in relation to the positive cultures.
| Exam | Culture + (n = 16) | Culture – (n = 164) | p | GAS + (n = 7) | GAS – (n = 173) | p |
|---|---|---|---|---|---|---|
| ASO Average (±DP) ≥ 200 U/Todd |
337.5 (102.42) 8 (50%) |
93.90 (15.92) 38 (23.1%) |
0.0001 0.04 |
657.1 (126.9) 7 (100%) |
93.64 (15.71) 39 (22.5%) |
0.0001 0.0001 |
* Student’s t-test and chi-squared test with Yates’ correction were applied.
ASO best value performance in relation to the GABHS.
| Exam | Value | S | Value | E | Best Accuracy | S/E | AUC |
|---|---|---|---|---|---|---|---|
| ASO (U/Todd) | 200 | 1 | 1200 | 1 | 200 | 1/0.8 | 0.93 |
* Values obtained by ROC curve. SPC: sensitivity TRP: specificity AUC: area under the curve.
The antistreptolysin-O titration average in patients that was identified in diverse Streptococcus groups was 337.5 Todd units, compared with 93.9 Todd units for patients with negative cultures (p = 0.0001). Among the 16 patients with positive culture, ASO was reagent (from 200 IU) in 8 (50%), and in 38 children that showed negative culture (23.1%), with p = 0.04. The average found on GABHS-present cases was 657.1 IU, compared with 93.94 IU on GABHS-absent cases ( p = 0.0001 ). 100% of patients that were infected showed the reagent ASO, a result found on 22.5% of non-infected patients (p = 0.0001). The highest value encountered was 1200 U/Todd. 39 participants (21.66%) presented ASO reagent without a positive oropharynx culture. In this group, the ASO titters ranged from 200 to 1000 U/Todd. Laboratory findings and its analysis can be seen on Table 3.
To stipulate the best ASO accuracy rate, a Receiver Operating Characteristic (ROC) curve was used, where the results from the 180 patients were computed. The best sensitivity to ASO was 200 Todd units, and the best specificity, 1200 units, providing the 200 units rate to be the best accuracy, with a sensitivity of 100% and specificity of 88% with 93% of patients under the created curve. The calculation summary made using the ROC curve can be found on Table 4.
DISCUSSION
According to this study, the oropharynx colonization prevalence of group A beta-hemolytic Streptococcus was estimated to be 3.9%. A similar study was conducted by Vieira et al. in São Paulo (SP) and Porto Velho (RO), but the patients were separated in two groups: children that went to preschool, and those who did not. In São Paulo, the prevalence was 8% in the group that went to preschool, and 2% in the other group. Porto Velho had a higher prevalence, 24% and 16%, respectively [19], which can be related to socioeconomics and environmental differences between these capitals. One reason why Ponta Grossa’s rate is intermediary (when compared with São Paulo’s group) could be the fact that this study used both children that went and did not go to preschool. Another study evaluated prevalence on 1061 asymptomatic children from Hawaii and American Samoa, finding prevalence of 3.4% and 13%, respectively [20]. It is known that in the summer there is a greater proportion of bacterial oropharyngitis (including those caused by GABHS). However, the seasonal difference was not evaluated in this study.
An interesting finding was the presence of group B Streptococcus (S. agalactiae) on 3 cultures, an agent responsible for urinary tract infection and neonatal meningitis. About one quarter of participants (25.5%) showed reagent ASO (superior to 200 Todd units), revealing a probable recent infection by GAS; also, a higher rate than the one found in a research made in Laranjal (PR) in 2005, which was 13.3% [21].
Pereira et al. showed the importance of vouching the GAS infection on the diagnosis of rheumatic fever, since only 24 to 29.6% of patients that fitted Jones’ criteria had documented infection.5 Another study made by Carvalho et al. found ASO titration elevated in 58.7% of patients with acute rheumatic fever [17]. Thereby, antistreptolysin-O’s sensitivity and specificity were widely evaluated in relation to rheumatic fever on different studies. Machado et al., while evaluating 78 cases of ARF, found a sensitivity of 73.3% and a specificity of 57.6% [22].
However, upon revising medical literature, no studies were found that evaluated those rates correlating ASO with oropharynx’s culture on gold-standard tests for GAS infection diagnosis, the positive cases ranged from 90 to 95% [23, 24].
The present study showed a qualitative and quantitatively significant relation between ASO and GAS presence (p = 0.0001 for both relation), and also ASO and Streptococcus (p = 0.04 and 0.0001, respectively).
Brazilian consensuses reinforce the need of regional studies to delimitate ASO cutoffs, in order to improve rheumatic fever diagnostic accuracy, as well as treatment or prophylaxis indication [8, 17].
Until now, this has been the first study to determinate ASO cutoff for its region, establishing a new GAE carrier approach algorithm, as seen on Fig. (1).
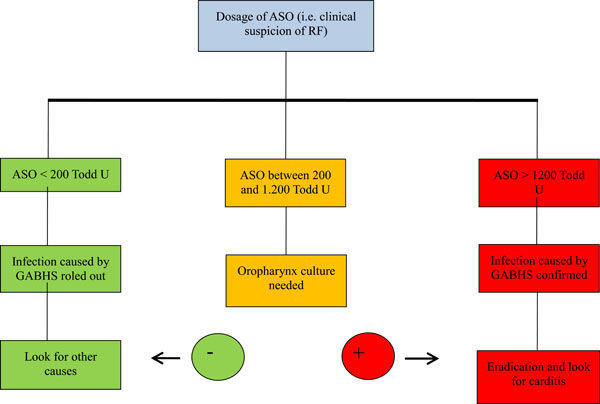
Approach to carriers of GABHS in front of ASO dosage.
While tabulating the ASO titration of participants in the ROC, a rate of 200 Todd units was found as being the best accuracy, showing 100% of sensitivity and 80% of specificity for GAS infection documentation. 93% of patients were found on the area under the curve (AUC) - an excellent result, since results higher than 70% are considered relevant [18]. Working with better sensitivity and specificity rates brings progress to public health, since it orients physicians toward the best way of investigating and the real needs of therapy [25]. Using antibiotic therapy in a rational matter, it is possible to maintain the high sensitivity to penicillin [26], as found in the study.
CONCLUSION
The results of this study indicate that the oropharynx colonization prevalence of group A beta-hemolytic Streptococcus (GABHS) found on Ponta Grossa (PR) was 3,9%. With the results that were found, in the region of Campos Gerais, patients with ASO equal to or higher than 1200 Todd units must receive oropharynx GAS infection treatment, since penicillin G benzathine treatment reduces cases of rheumatic fever [12] by 80%. More studies must be made with different segments of the population, once the ASO rate can change from one area to the other [27, 28].
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


