Targeted Therapy with Rituximab in Felty’s Syndrome: A Case Report
Abstract
Felty’s syndrome is a rare, severe extra-articular manifestation of rheumatoid arthritis (RA). There is no standard therapy, and several disease-modifying antirheumatic drugs have been used with varying success. Only very few reports exist in literature on the use of biologicals in this indication and this with a variable efficacy. We report the case of a 53-year-old woman with severe refractory/partly undertreated RA who presented with Felty’s syndrome and pancytopenia, in whom treatment with rituximab led to an marked increase of red blood cells, neutrophils and thrombocytes. In addition, the RA disease activity status improved dramatically and treatment with steroids could be reduced. The current sparse literature on this topic is reviewed.
INTRODUCTION
Felty’s syndrome is a rare but severe extra-articular manifestation of rheumatoid arthritis (RA), in most cases developing after a longer refractory disease course. The syndrome is defined as a triad of RA, neutropenia and splenomegaly [1]. Due to recurrent infections, patients with Felty’s syndrome have an increased mortality, with a 5-year mortality rate of up to 36% [2, 3]. To treat Felty’s syndrome, various disease-modifying antirheumatic drugs have been used with mixed success and few data are available on the use of biologicals that might even increase the infection risk. Because of the rarity of the disease, definite efficacy and safety results from biological therapies are not expected to become available in the future. Few case reports in the literature deal with the use of rituximab which might be seen as a preferred treatment in RA patients with major systemic features. We report on a severe refractory and partly undertreated RA patient with Felty’s syndrome and discuss her successful treatment with rituximab. Oral consent was obtained from the patient to report her evolution anonymously.
CASE REPORT
A 53-year old Caucasian woman with a 22-year history of refractory rheumatoid factor and CCP positive RA was referred to our University Hospital in October 2010 because of a 2-year history of pancytopenia and hepatosplenomegaly, diagnosed as Felty’s syndrome and a very severe disease activity status of her RA. In addition, she was known with arterial hypertension and an allergy to penicillin. She smoked >30 pack years but stopped smoking a few years ago. Epilepsy was diagnosed in 1992 after a generalized tonic-clonic seizure, for which she was treated with phenytoin and primidone. Previous therapies for RA consisted of sulphasalazine and intermittent glucocorticoids. Methotrexate in recent years was never considered because of pancytopenia. A bone marrow biopsy was performed in the referring hospital in 2009, showing only mild dysmegakaryocytosis and a modest decrease of the white blood cell maturation. Treatment with sulphasalazine was temporarily interrupted because of this pancytopenia. Because of persisting pancytopenia, hepatosplenomgaly and enlarged axillary and mediastinal lymph nodes on computer tomography of the chest, a lymph node biopsy was done beginning of 2010 which showed follicular hyperplasia and no evidence of lymphoma. Meanwhile she was on oral glucocorticoid therapy but a severe polyarthritis persisted and purpura on the lower limbs appeared clinically compatible with rheumatoid vasculitis. Treatment with topical steroids and hydroxychloroquine was added, the dose of glucocorticoids was increased and the patient was referred to our third care referral centre.
She presented in our hospital with an active destructive polyarthritis and large RA nodules, with a disease activity score (DAS28) of 4.7 while on methylprednisolone 16 mg, hydroxychloroquine 200mg, sulphasalazine 2000mg and piroxicam 20mg daily. In addition, she was treated with phenytoin and primidon for epilepsy, acebutalol and furosemide for hypertension, pantoprazol for oesophagitis as well with escitalopram 10mg and lormetazepam. Her spleen was enlarged (17x11 cm), as well as her liver (19 cm craniocaudal diameter). She had purpura on the lower limbs and a diffuse lymphadenopathy, with follicular hyperplasia on biopsy. Study of her files revealed a white cell count remaining persistently less than 1.0x109/l and a hemoglobin level below 10.9 g/dl. Thrombocytes decreased continuously during the last year, and were less than 20x109/l at presentation in our hospital. A new bone marrow biopsy showed some hypocellularity without dysplasia, no blast cells and a normal central lymphocytosis without atypia. Immunophenotyping showed no monoclonal B- or T-cell process. Hydroxychloroquine and sulphasalazine were stopped. Methylprednisolone was maintained at 16mg daily and a treatment with rituximab was started twice 1000mg over 2 weeks in November 2010, and again twice 1000mg (588 mg/m2) in July 2011. DAS score dropped from 4.7 to 3.0, 17 months after treatment start. CRP decreased from 41 mg/L to 7 mg/L, and the erythrocyte sedimentation rate (ESR) decreased from 73 mm/h to 32 mm/h. An overview of the evolution of DAS and the pancytopenia in the first year of follow up is presented in Fig. (1). The number of red blood cells, neutrophils and thrombocytes increased significantly: hemoglobin from 10.9 g/dl to 15.1 g/dl, white blood cells from 0.81 x 109/L to 3.92 x 109/L and thrombocytes from 18 x 109/L to 120 x 109/L. Interestingly the hypergammaglobulinemia dropped from 23.9 g/l at treatment start to 19.2 g/l and 15.8 g/l, 6 and 18 months after treatment initiation. Patient was satisfied as also pain, general well being and quality of life dramatically improved. From month 4 on gradually tapering of glucocorticoids was initiated and 1 year after start of rituximab she was on 6mg daily. There were no infections within the first treatment year and planned foot surgery because of severe deformities could be performed without any problem.
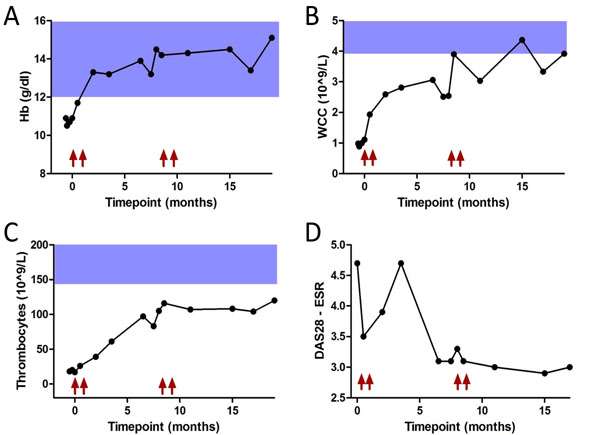
The evolution of hemoglobin (Hb) (A), white cell count (WCC) (B), thrombocytes (C) and the DAS28 score (D) up to 19 months after therapy start. Arrows indicate time of Rituximab infusions.
DISCUSSION
We report an important beneficial effect of rituximab in a patient with Felty’s syndrome, without immediate side effects. Both the arthritis and the hematological features of the syndrome were much better controlled by 4 months after therapy start and steroid tapering was successful. The purpura on the lower limbs did not reoccur and there were no infectious complications.
Rituximab is chimeric monoclonal antibody against CD20, which is expressed on the surface of B-lymphocytes, causing B-cell depletion. It was originally developed for the treatment of B-cell lymphoma [4, 5]. As T-cell activation is classically described as the key component of the pathogenesis of rheumatoid arthritis (RA), the efficacy of rituximab in RA came initially as a surprise. In 1999 Protheroe et al. wrote a case in which a patient with erosive arthritis was treated with rituximab for a B-cell lymphoma [6]. Following this treatment, the patient unexpectedly became free of musculoskeletal symptoms and joint inflammation. The role for B-cells in RA was later confirmed in randomised controlled studies, in which response rates of 51% and 85% were noticed with rituximab [7, 8].
While the immunopathogenesis of RA becomes further unraveled, Felty’s syndrome remains still a mystery. Traditionally, splenomegaly was seen as a feature of systemic inflammation, causing neutropenia by sequestration and justifying splenectomy as treatment. When Gupta et al. described reduced colony-stimulating activity in the serum of patients with Felty’s syndrome [9], a role for impaired regulation of granulopoiesis became recognized in addition to peripheral destruction or sequestration (hypersplenism) as cause of neutropenia. As Felty’s syndrome is an autoimmune disease, the loss of immunologic tolerance to self-antigens can cause neutropenia through both peripheral immunological destruction and impaired granulopoiesis. This is traditionally described as a cell-mediated destruction. However, humoral immunity might be important as well in causing neutropenia. Humoral suppression of normal granulopoiesis has been reported [10], as well as serum antibodies against mature neutrophils [11]. The antibody production against circulating neutrophils and against granulopoiesis can be suppressed by B-cell depletion, providing a rationale for the use of rituximab. However, the role of B-cells in autoimmune cytopenias can not only be limited to the production of antibodies, as the efficacy of rituximab in certain conditions associated with antibodies, is not correlated with a reduction of these antibodies [12]. They could also play a role through the production of cytokines and by acting as antigen-presenting cells supporting the activation of autoreactive cells [13].
The use of rituximab in patients with Felty’s syndrome is rarely mentioned in the literature. Weinreb et al. [14], Salama et al. [15] and Lekharaju et al. [16] described a beneficial response. In contrast, Sordet et al. described two cases of Felty’s syndrome where rituximab lacked efficacy [17]. These varying results underscore the multifactorial pathogenesis of Felty’s syndrome and could suggest the existence of subsets of patients where rituximab could be beneficial [16]. The exact role of rituximab in Felty’s syndrome needs further investigation. In this particular case it should also been noted that methotrexate was never used in the past because of fear for the hematological status. In our opinion and according to literature, this therapy option might also be effective in Felty syndrome and whenever her disease would relapse in the future the addition of methotrexate will be considered [18].
CONCLUSION
This observation contributes in the absence of randomized controlled data to the understanding of the role of specific biological therapies as rituximab in rare immune mediated disorders and appeals to further collaborative studies to increase robustness of data in these challenging situations.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENTS
Declared none.


