All published articles of this journal are available on ScienceDirect.
Fibromyalgia Concomitant with Seropositive Rheumatoid Arthritis in a Tertiary Hospital in South-Western Saudi Arabia: Prevalence and Treatment Patterns
Abstract
Introduction:
Rheumatoid arthritis (RA) patients with fibromyalgia syndrome (FMS) report worse functional status and quality of life hence the association has important clinical implications. FMS can be challenging to treat, and the current evidence recommends a multidisciplinary treatment approach focused on symptom management.
Aim:
Information regarding the current prevalence of FMS in RA patients is lacking. Thus, this study aims to address the prevalence and predictors of FMS in seropositive RA patients and demonstrate our clinical practice in the management of FMS.
Methods:
Participants’ data was gathered from Aseer central hospital (ACH) rheumatology clinics and daycare units over a period of 2 years. Subjects were assessed using the 2010 American College of Rheumatology (ACR) criteria for FMS. Data were collected from medical records, including patient demographics, comorbidities and concomitant FMS-related data.
Results:
Out of 310 seropositive RA patients, 15% (n = 47) fulfilled the diagnostic criteria for FMS. Of them, 29, 11 and 7 were on pregabalin, amitriptyline and duloxetine, respectively. Half of FMS patients showed one or more therapy changes. A significant difference between RA patients with and without concomitant FMS was observed, including age, gender and comorbidities.
Conclusion:
In this retrospective study, a high prevalence of FMS in individuals with seropositive RA was identified. This study explores real-world practice in the treatment of FMS with remarkable findings regarding underdosing and lower discontinuation rate of pregabalin.
1. INTRODUCTION
Fibromyalgia is a syndrome of unknown etiology that causes widespread chronic musculoskeletal pain, fatigue, functional and somatic symptoms [1]. The estimated prevalence of FMS is approximately 2-3% in the general population, with age peaking at 50–60 years old 3 and a female: male ratio of 3:1 [2-4]. The presence of FMS in several rheumatic diseases with a structural pathology has been reported as 11–30% [5] and was found to be 12-48% in patients with RA particularly [6, 7]. FMS adversely affects RA patients leading to increased pain, fatigue and consequently, higher estimation of disease activity [8]. Therefore, the association between RA and FMS provides important implications in clinical practice.
The diagnosis of FMS depends on documenting a number of subjective symptoms like generalized pain, which is reported in 20–30% of patients, and physical or mental fatigue with various degrees of sleep disturbance [9]. Other symptoms include autonomic disturbance in the form of orthostatic hypotension, Raynaud phenomenon, blurred vision, photophobia and xerostomia, and regional pain syndromes such as migraine, irritable bowel syndrome and dysmenorrhea [10, 11].
The therapeutic approach to managing patients with FMS is characterized by integrated and multidisciplinary interventions through patient education, fitness, pharmacological treatment and psychotherapy [12]. The latest European League Against Rheumatism criteria (EULAR) recommendations on FMS management emphasize the importance of initial use of non-pharmacological measures, but the only ‘strong’ recommendation is in favor of aerobic exercise as it can improve pain and physical function in patients with FMS [12].
The U.S. Food and Drug Administration (FDA) has approved three drugs for the treatment of FMS. They include serotonin–norepinephrine reuptake inhibitors (SNRI): (duloxetine and milnacipran) and anticonvulsant (pregabalin) [13]. Moreover, systematic literature reviews and meta-analyses suggest that tricyclic antidepressant amitriptyline is effective at treating FMS, especially in reducing pain and fatigue [14, 15]. The recommended dosages for approved treatments are 60 mg per day for duloxetine, 300 mg per day for pregabalin (up to 450 mg per day), 100 mg per day for milnacipran (up to 200 mg per day) and up to 75mg/day for amitriptyline with starting dose of 25 to 50 mg bedtime [13-15].
The most common adverse effects (AEs) with SNRI are nausea, headache, dry mouth, insomnia, fatigue, constipation, diarrhea, and dizziness. While typical AEs of pregabalin are dose-dependent, which may include sedation, dizziness, peripheral edema, nausea and weight gain [16, 17]. Dry mouth, constipation, fluid retention, weight gain, and difficulty concentrating are common with tricyclic antidepressant [15].
Non-pharmacological therapies are included in the EULAR recommendations and might be considered as adjunctive treatment for many patients [12]. Cognitive-behavioral therapy, balneotherapy, tai chi, and yoga are methods that can be used in conjunction with patient education and pharmacological treatment to improve outcomes and relieve symptoms [18-20].
The aim of this study is to address the prevalence and determinants of FMS in patients with seropositive RA in Aseer Central Hospital in South-Western Saudi Arabia, and to describe our current FMS treatment patterns.
2. MATERIALS AND METHODS
A record-based retrospective study was conducted on 1000 consecutive patients diagnosed with RA according to the 2010 ACR/EULAR criteria. They attended the outpatient clinics and the daycare unit in a tertiary care hospital in South-Western Saudi Arabia from June 2018 to June 2020.
The inclusion criteria included adults older than 18 years with either RF or anti-CCP positivity, apart from patients who lost follow-up or had incomplete clinical data.
This research protocol was approved by the Aseer General Directorate of Health Affairs-Regional Committee for Research Ethics (IRB Registration No: H-06-B-091). The study has therefore been performed in accordance with the Declaration of Helsinki. Patients’ clinical data were used only for research purposes while maintaining the confidentiality of patient’s records throughout the study.
The medical records of 310 patients who met the inclusion criteria were reviewed, and data were extracted about patient demographics and characteristics, including RA duration, extraarticular manifestations and other chronic comorbidities including hypertension (HTN), diabetes mellitus (DM), ischemic heart disease (IHD), malignancies, gastrointestinal diseases, infections, lung disease, osteoporosis and hypothyroidism. Evidence of concomitant FMS – according to 2010 ACR criteria – was retrieved from the patient's files. The ACR 2010 criteria for FMS include the widespread pain index (WPI), measuring the number of painful body regions, and the symptom severity score (SS), which evaluates associated FMS symptoms. The diagnosis of FMS is supported when a WPI of ≥ 7 and SS ≥ 5, or WPI 3–6 and SS ≥ 9 are met [21]. A review of FMS patients’ files was conducted to document: the initial treatment regimen, switching between different medications, and drug discontinuation. For patients who were diagnosed with FMS, all follow-up visits were reviewed to document the following: initial treatment regimen, switching between different medications of FMS and drugs discontinuation.
2.1. Data Analysis
The data was collected, reviewed and entered in Statistical Package for Social Sciences version [21] (SPSS: An IBM Company). All statistical methods used were two-tailed with an alpha level of 0.05, considering significance if the P value is less than or equal to 0.05. Descriptive analysis was done based on frequency and percent distribution of patients’ bio-demographic data according to their FMS diagnosis including age, gender, duration and treatment of RA, co-morbidities, initial and final clinical disease activity index (CDAI). The distribution of FMS predictors among RA patients was assessed using cross-tabulation. Pearson chi-square test was used to test for relations significance.
3. RESULTS
The study included 310 patients, out of them 47 (15%) were diagnosed with FMS and 263 (85%) were free of FMS.
The demographic characteristics of the patients are summarized in Table 1.
Table 1.
| Bio-demographic data | The Patient Diagnosed with FMS | |||
| Yes | No | |||
| No. | % | No. | % | |
| Age in years | ||||
| 21-30 | 0 | 0.0% | 18 | 6.8% |
| 31-40 | 2 | 4.3% | 51 | 19.4% |
| 41-50 | 6 | 12.8% | 64 | 24.3% |
| 51-60 | 21 | 44.7% | 78 | 29.7% |
| 61+ | 18 | 38.3% | 52 | 19.8% |
| Gender | ||||
| Male | 2 | 4.3% | 35 | 13.3% |
| Female | 45 | 95.7% | 228 | 86.7% |
| Co-morbidities | ||||
| Yes | 34 | 74.4% | 133 | 46.8% |
| No | 13 | 25.6% | 140 | 53.2% |
| Co-morbidities | ||||
| HTN | 14 | 30% | 62 | 24% |
| DM | 15 | 32% | 46 | 17% |
| IHD | 1 | 2% | 10 | 4% |
| Osteoporosis | 11 | 23% | 30 | 11% |
| Hypothyroidism | 13 | 28% | 47 | 18% |
| Duration of RA | ||||
| 1-4 | 6 | 12.8% | 49 | 18.6% |
| 5-9 | 20 | 42.6% | 112 | 42.6% |
| 10-19 | 14 | 29.8% | 81 | 30.8% |
| 20+ | 7 | 14.9% | 21 | 8.0% |
| CDAI at the time of presentation | ||||
| Moderate | 1 | 2.1% | 11 | 4.2% |
| High | 46 | 97.9% | 252 | 95.8% |
| Current RA treatment regimen | ||||
| csDMARDs | 15 | 31.9% | 121 | 46.0% |
| bDMARDs | 32 | 68.1% | 142 | 54.0% |
| Current CDAI | ||||
| Remission | 1 | 2.1% | 133 | 50.6% |
| Low | 19 | 40.4% | 124 | 47.1% |
| Moderate | 27 | 57.4% | 6 | 2.3% |
Eighty-three percent of RA patients with FMS aged above 50 years compared to 49.5% of patients without FMS. In both groups, the majority of patients were females, with more female predominance in the FMS group (95.7%) compared to 86.7% of the comparison group. Chronic comorbidities were detected in 25.5% of RA patients with concomitant FMS. Considering the duration of RA, it was similar between the two groups and the initial CDAI was high in both groups. Biological disease-modifying antirheumatic drugs (bDMARDs) use was higher in the FMS group representing 68% of the patients compared to 54% in patients without FMS. Among patients with FMS, remission was achieved in only 2.1% compared to 50.6% in the other group. Moderate disease activity was detected in 57.4% of those with FMS compared to 2.3% in those without FMS.
With regards to predictors of FMS in RA patients, the prevalence of FMS increased with age with a recorded statical significance (P=.001). FMS was significantly higher among female patients than in males (P=.048). DM was diagnosed in 26.2% of RA patients who have FMS compared to 12.4% of those without FMS (P=.007). The presence of chronic medical illnesses was significantly higher in patients with FMS, hence only 8.5% of FMS patients were not diagnosed with any comorbidities (P=.005). No group differences were found regarding other factors, including duration of RA, type of RA treatment regimen, initial CDAI, and other systematic manifestations of RA (Table 2).
Regarding treatment regimen, pregabalin was the predominant first prescribed drug representing 70%, followed by amitriptyline in 19% then duloxetine in 11% of the cases. The current treatment regimen for FMS is detailed in Table 3.
| Factors | The Patient Diagnosed with FMS | p-value | ||||
| Yes | No | |||||
| No. | % | No. | % | |||
| Age in years | 21-30 | 0 | 0.0% | 18 | 100.0% | .001* |
| 31-40 | 2 | 3.8% | 51 | 96.2% | ||
| 41-50 | 6 | 8.6% | 64 | 91.4% | ||
| 51-60 | 21 | 21.2% | 78 | 78.8% | ||
| 61+ | 18 | 25.7% | 52 | 74.3% | ||
| Gender | Male | 2 | 5.4% | 35 | 94.6% | .048* |
| Female | 45 | 16.5% | 228 | 83.5% | ||
| Prescence of DM | Yes | 16 | 26.2% | 45 | 73.8% | .007* |
| No | 31 | 12.4% | 218 | 87.6% | ||
| Number of other co morbidities | None | 13 | 8.5% | 140 | 91.5% | .005*$ |
| 1 | 14 | 18.4% | 62 | 81.6% | ||
| 2 | 13 | 22.4% | 45 | 77.6% | ||
| 3 | 4 | 23.5% | 13 | 76.5% | ||
| 4 | 3 | 50.0% | 3 | 50.0% | ||
| Duration of RA | 1-4 | 6 | 10.9% | 49 | 89.1% | .408 |
| 5-9 | 20 | 15.2% | 112 | 84.8% | ||
| 10-19 | 14 | 14.7% | 81 | 85.3% | ||
| 20+ | 7 | 25.0% | 21 | 75.0% | ||
| CDAI at the time of presentation | Moderate | 1 | 8.3% | 11 | 91.7% | .501 |
| High | 46 | 15.4% | 252 | 84.6% | ||
| Current RA treatment regimen | bDMARDs | 32 | 18.4% | 142 | 81.6% | .073 |
| csDMARDs | 15 | 11.0% | 121 | 89.0% | ||
| Presence of deformity | Yes | 2 | 9.1% | 20 | 90.9% | .410 |
| No | 45 | 15.6% | 243 | 84.4% | ||
| Extra articular lung manifestations | Yes | 7 | 24.1% | 22 | 75.9% | .157 |
| No | 40 | 14.2% | 241 | 85.8% | ||
| Extra articular skin manifestations | Yes | 0 | 0.0% | 5 | 100.0% | .341$ |
| No | 47 | 15.4% | 258 | 84.6% | ||
| Associated Sjorgen’s syndrome | Yes | 3 | 37.5% | 5 | 62.5% | .074 |
| No | 44 | 14.6% | 258 | 85.4% | ||
| Hematological manifestations | Yes | 8 | 14.3% | 48 | 85.7% | .840 |
| No | 39 | 15.4% | 215 | 84.6% | ||
| Patient on steroid | Yes | 19 | 13.1% | 126 | 86.9% | .571 |
| No | 28 | 17.1% | 137 | 82.9% | ||
Abbreviations: RA, rheumatoid arthritis; FMS, fibromyalgia syndrome; DM, diabetes mellites; CDAI, the clinical disease activity index; csDMARDs, conventional disease-modifying antirheumatic drugs; bDMARDs, biological disease-modifying antirheumatic drugs.
| Clinical Data | No. | % |
| Duration of FMS | - | - |
| 1-4 | 21 | 45% |
| 5-10 | 26 | 55% |
| Current FMS treatment | - | - |
| Pregabalin | 29 | 62% |
| Amitriptyline | 11 | 23% |
| Duloxetine | 7 | 15% |
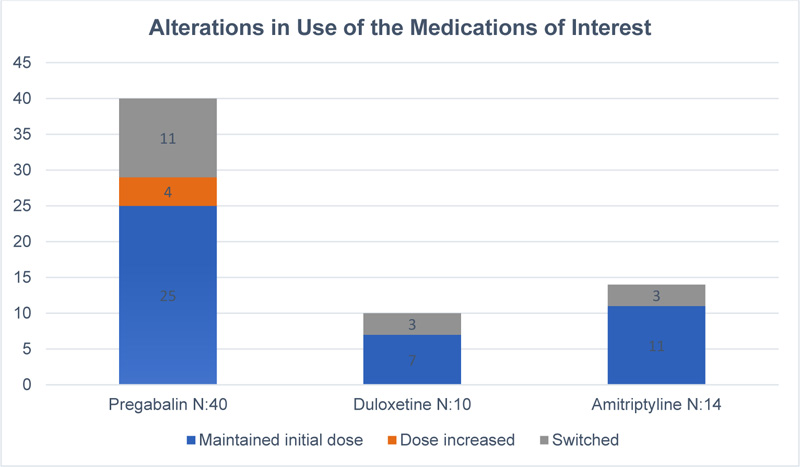
Almost half of FMS patients showed one or more therapy changes. Another relevant observation in this study is that only 5 patients received the recommended dose of pregabalin while the rest received lower doses.
Regarding the discontinuation rate of medications, 29% of pregabalin-treated, 42% of duloxetine treated, and 35% of amitriptyline-treated patients discontinued their medications. The two most common reasons for treatment discontinuation were the occurrence of adverse events followed by lack of effectiveness.
Information regarding switching and dose alteration for each drug since time of prescription is shown in Fig. (1).
4. DISCUSSION
This is the first regional study to estimate the prevalence of FMS in seropositive RA patients. In our study, 15% of patients with RA had concomitant FMS, which is similar to findings in previously published studies [6, 7, 22].
With regards to participants’ demographics, 83% of FMS patients were aged above 50 years.
In this study, there was a notable association between the female gender and the presence of FMS, confirming the results of previous data [4]. Our findings suggest that older age, female gender and the presence of a high number of comorbidities especially DM increase the likelihood of FMS development. Wolfe, Frederick, et al. examined predictors of FMS development in RA patients. The following factors contribute to the development of FMS: longer RA duration, marital status (divorced, widow), household income below median, poverty status, smoking, obesity, comorbid conditions, prednisone and opioid use [7]. A cross-sectional study found no group differences regarding disease duration, age, gender, and serological status [23]. In contrast to the published data, we didn’t find a significant impact on disease duration or the baseline disease activity.
Concomitant FMS likely influences disease activity measures [6]. In our study, more than half of the patients with concomitant FMS did not achieve the RA treatment target of remission or low disease activity. However, no statistically significant difference was found in the use of biological treatment in both groups.
A significant number of our patients using pregabalin were on much lower doses than recommended, possibly due to concerns about adverse effects. Our findings on pregabalin underdosing were consistent with a cohort examining dosing patterns on the 3 FDA-approved medications in patients with FMS in which 89% of patients received <300 mg daily of pregabalin [24].
In comparison to real-world studies, we have a higher discontinuation rate of duloxetine (425) and amitriptyline (35%). Interestingly, pregabalin has the lowest discontinuation rate (29%). In placebo-controlled trials, up to 20-22% of patients discontinued approved therapies because of AEs [18, 25]. In another observational study, 48% of pregabalin-treated and 42% of duloxetine-treated patients discontinued their medications at 12 months due to intolerable AEs and lack of effectiveness 26. For amitriptyline, eight placebo-controlled trials reported the total number of dropouts due to AEs caused by amitriptyline reached 12% [26].
Limitations to our study may include the small sample study group, making it difficult to estimate associations with greater precision. Also, due to the study being retrospective, we lacked the ability to ascertain the reasons behind nonadherence to the treatment. Milnacipran was not included in the study as it is not available in our hospital. Furthermore, the use of nonpharmacologic treatment was not assessed; future studies would be needed to examine this issue.
CONCLUSION
A high prevalence of FMS in individuals with seropositive RA was identified. Our findings provide important information about FMS treatment patterns. Pregabalin users were most likely to receive a lower than recommended dose and in contrast to real-world studies, they were the least likely to discontinue the treatment. The results suggest medication change is common, possibly due to dissatisfaction with initial treatment. Further research is needed to investigate the causes for the lack of adherence to prescribed medicine and high discontinuation rates.
LIST OF ABBREVIATIONS
| RA | = Rheumatoid Arthritis |
| FMS | = Fibromyalgia Syndrome |
| ACH | = Aseer Central Hospital |
| ACR | = American College of Rheumatology |
| EULAR | = European League Against Rheumatism |
| SNRI | = Serotonin and Norepinephrine Reuptake Inhibitors |
| AEs | = Adverse Effects |
| DM | = Diabetes Mellitus |
| WPI | = Widespread Pain Index |
| SS | = Severity Score |
| CDAI | = The Clinical Disease Activity Index |
| csDMARDs | = Conventional Disease-modifying Antirheumatic Drugs |
| bDMARDs | = Biological Disease-modifying Antirheumatic Drugs |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This research protocol was approved by Aseer General Directorate of Health Affairs-Regional Committee for Research Ethics (IRB Registration No: H-06-B-091).
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All the humans were used in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013 (http://ethics.iit.edu/ecodes/node/3931).
CONSENT FOR PUBLICATION
Patients’ clinical data was used only for research purposes while maintaining confidentiality of patient’s records throughout the study.
STANDARDS OF REPORTING
STROBE guidelines were followed.
AVAILABILITY OF DATA AND MATERIALS
The authors confirm that the data supporting the findings of this study are available within the manuscript.
FUNDING
The authors did not receive any financial support for this work.
CONFLICT OF INTEREST
The authors report no conflicts of interest in this work.
ACKNOWLEDGEMENTS
Declared none.


