All published articles of this journal are available on ScienceDirect.
COVID-19 Prevalence and Outcomes among Individuals with Rheumatoid Arthritis and Systemic Lupus Erythematosus Taking Hydroxychloroquine; A Retrospective Analysis
Abstract
Introduction:
The SARS-CoV-2 global pandemic has resulted in a universal search for potential treatments of Coronavirus Disease 2019 (COVID-19). Initial reports of the therapeutic potential of chloroquine (CQ) and hydroxychloroquine (HCQ) and early non-randomized non-controlled studies were followed by subsequent trials refuting such properties. The use of CQ and HCQ in diseases, such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE), prompted us to examine the prevalence of COVID-19 and proposed prophylactic and therapeutic properties of HCQ in this population.
Methods:
A total of 103 patients with RA and SLE aged 18 to 75 diagnosed with COVID-19 were identified. The patients were categorized as those taking HCQ (cases) and those not on HCQ (controls) for at least 6 months. Primary (mechanical ventilation, length of stay, death) and secondary outcomes were defined, data were collected, and results were compared and statistically analyzed between cases and controls.
Results:
No statistical difference was observed in demographic features, baseline comorbidities, and medications. Primary outcomes’ statistical analysis did not reveal any differences between cases and controls. Statistical analysis of secondary outcomes revealed that cases had a statistically higher chance of being tachypneic (p 0.034). D-Dimer (p 0.017) and LDH levels (p 0.044) were found to be significantly lower in cases versus controls.
Conclusion:
This study highlights the lack of clinical prophylactic and therapeutic efficacy of HCQ against COVID-19 when taken at regular doses for patients with RA and SLE. It also shows that the prevalence of COVID-19 was similar in RA and SLE patients regardless of baseline consumption of HCQ.
1. INTRODUCTION
The coronaviruses are positive-stranded RNA viruses that usually lead to mild upper respiratory tract infections without complications [1]. In the early 21st century, new species of this family, Severe Acute Respiratory Syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome coronavirus (MERS-CoV), emerged [2], causing severe respiratory disease. SARS-CoV-2, a third novel coronavirus and the causative agent of coronavirus disease 2019 (COVID-19), emerged from Wuhan, China, in late 2019 and led to an ongoing, challenging worldwide pandemic in 2020 [1]. A global attempt to develop pharmacotherapeutics and vaccines has ensued since the emergence of SARS-CoV-2. The most debated and presumed initially effective medications for COVID-19 are the antimalarial agents chloroquine (CQ) and hydroxychloroquine (HCQ).
While the anti-inflammatory and anti-malarial properties of these drugs have long been recognized, their potential use for the management of Rheumatoid Arthritis (RA) and Systemic Lupus Erythematosus (SLE) was quite incidental [3]. Nevertheless, they now comprise a cornerstone in the management of autoimmune diseases [3, 4]. The immunomodulatory effects of these medications have been further elucidated through a variety of studies, with these medications shown to inhibit endosomal maturation by abrogating endosomal acidification resulting in inhibition of proteolysis, glycosylation, antigen presentation, and innate and adaptive immune mechanisms by the inhibition of Toll-like receptor signaling, modulation of the production of inflammatory cytokines, Interleukin-1 (IL-1), Interleukin-6 (IL-6), and Tumor Necrosis Factor-alpha (TNF-alpha) by macrophages/monocytes, and inhibition of T- and B-cell signaling [3-5]. Given these anti-inflammatory properties, it is hypothesized that these medications could inhibit initial viral infection, the resultant inflammatory cascade, and viral release via reduced acidification of endocytic vesicles leading to reduced viral infectivity [6-9]. In the case of these novel coronaviruses, glycosylation of the cell-surface receptor ACE2 seems to be important in viral docking and entry. HCQ inhibition of ACE2 glycosylation is associated with reduced infection in in vitro models and was also found to be effective not only in treating infection but also in preventing infection, highlighting the potential prophylactic use of chloroquine and HCQ in SARS-CoV-1 [10] and subsequently SARS-CoV-2 [11-13]. Influenced by this data and the global search of treatment for COVID-19, Gautret and colleagues reported a non-randomized clinical trial that demonstrated increased SARS-CoV-2 clearance with HCQ [14]. Despite widespread initial acceptance, several key issues with the study were raised, including a small sample size and discrepancies between the control and treatment groups, among others. Several follow-up studies by the same group as well as others reported clinical benefit [14, 15], while separate studies found no significant benefit with HCQ in COVID-19 [16-22].
Individuals with autoimmune disease, specifically RA and SLE, provide a unique lens by which HCQ and its effect on SARS-CoV-2 pathogenesis, infectivity, and the outcome can be evaluated. Considering controversies and the ongoing global debate regarding the efficacy and safety of chloroquine and HCQ as a prophylactic and therapeutic option for COVID-19, we proposed to examine the prophylactic and therapeutic efficacy of HCQ in individuals with RA or SLE on HCQ across the MedStar Health system and evaluate the incidence and outcomes of SARS-CoV-2 infection in this specific population.
2. METHODOLOGY
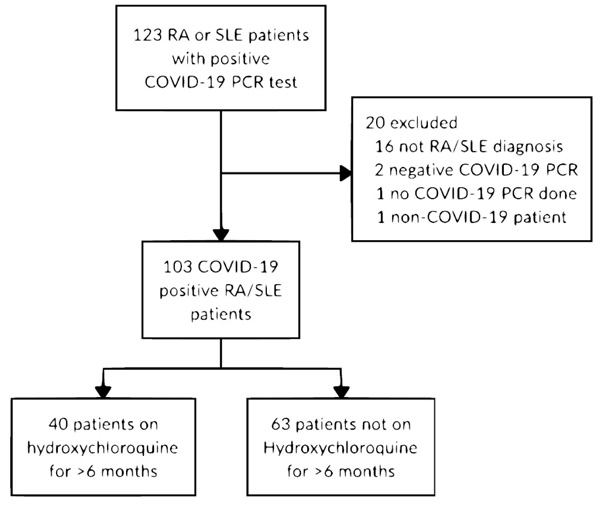
Patients diagnosed with RA or SLE, aged 18 - 75, across all inpatient and outpatient MedStar Health facilities were identified. Only those that were diagnosed with COVID-19 via a positive SARS-CoV-2 PCR assay were subsequently selected. The date range for inclusion was March 1, 2020, to June 15, 2020. Two comparison groups were subsequently made: 1) individuals actively taking HCQ at the time of the study (cases), and 2) individuals not taking HCQ at the time of the study (controls) (Fig. 1). Patients that were taking HCQ for at least 6 months, previously defined as the duration to reach a steady-state [23], were labeled as cases. Dosage, frequency, and whether HCQ was continued if admitted to the hospital were also noted. Other chronic medications, such as diabetic medications, beta-blockers, and angiotensin-converting enzyme inhibitors (ACE)/angiotensin II receptor blockers (ARBs), as well as additional immunomodulators (colchicine, belimumab, etanercept (Enbrel), methotrexate, Enbrel, sulfasalazine), were recorded.
Demographics and COVID-19 associated features including the presence of symptoms (fever, cough, sputum production, shortness of breath, tachypnea, tachycardia, diarrhea, nausea, and vomiting) and medical comorbidities (smoking, asthma, hypertension, hyperlipidemia, COPD, CKD, obesity, diabetes mellitus, etc.) were analyzed. A respiratory rate of greater than 20 breaths per minute was considered tachypnea, and a heart rate of greater than 100 beats per minute was considered tachycardia. To eliminate possible charting errors, we considered 2 or more recordings of each tachycardia and tachypnea to be counted as the true presence of these symptoms. The complete list of medical comorbidities and symptoms is given in Table 1, respectively.
Other COVID-19 and patient-associated factors were categorized into primary and secondary outcomes. We defined primary outcomes as the need for mechanical ventilation, hospital length of stay, and death. Secondary outcomes were defined as symptoms and signs upon presentation, radiological findings, primary laboratory data, and other COVID-19 associated complications such as admission to hospital, admission to intensive care unit, hypoxia, acute kidney injury, elevation in liver enzymes, and acute respiratory distress syndrome. Radiological findings were further categorized into three groups of bilateral infiltrates, bilateral opacities, and ground-glass opacities. Hypoxia was defined as the need for a nasal cannula or a SpO2 less than 92%, whereas sepsis was defined by a qSOFA score equal to or greater than 2. Statistical analyses were performed using mean and standard deviations for continuous variables and frequency and percentages for categorical variables. Comparisons of the means of the two groups, cases, and controls, were made using two samples t-test. Interquartile range was used to determine the spread of data points. Proportions of the two groups were compared using the chi-square test or Fisher’s exact test. Some p-values were not performed due to small sample sizes.
3. RESULTS
Among the 123 patients that were identified, 20 patients failed to either meet the inclusion criteria or were falsely included and were subsequently excluded from the study (Fig. 1). A total of 103 SARS-CoV-2 PCR positive RA and/or SLE patients were subsequently identified and selected for this retrospective observational study. After data collection and appropriate categorization, patient-specific features such as demographics, baseline medications, signs and symptoms on presentation, and primary and secondary outcomes were compared and analyzed between cases and controls. Overall, the study population included 84 (81.6%) females and 19 (18.4%) males. There were 40 (38.8%) and 63 (61.2%) patients in the cases and control groups, respectively. The mean age of cases was 53.3 years (SD: 15.9), comprised of 17 (42.5%) patients with RA, 22 (55%) patients with SLE, and 1 (2.5%) patient with both RA and SLE, while the mean age of controls was 64.6 years (SD: 14.3), comprised of 53 (84.1%) patients with RA and 10 (15.9%) patients with SLE (Table 1). Medications such as beta-blockers, angiotensin-converting enzyme inhibitors, and diabetic medications were also analyzed without any meaningful trend or difference between the two groups (Table 1). RA and SLE-specific medications other than HCQ were also reviewed and analyzed. Steroids and methotrexate comprised the most frequently utilized adjunctive therapeutics, while other adjunctive agents included sulfasalazine, etanercept, colchicine, and belimumab. Cases were more likely to be on belimumab and less likely to be on methotrexate with p values of 0.021 and less than 0.001, respectively.
Primary outcomes, as defined in the methods section, were compared and analyzed for any statistical difference between the cases and control groups. No statistically significant differences were identified for primary outcomes (Table 2).
Chest radiographs and computed tomography from the day of presentation were analyzed with findings categorized into three groups, including bilateral infiltrates, bilateral opacities, and ground-glass opacity compared between cases and controls. There were no significant differences observed in the cases versus controls. Laboratory diagnostics that have been previously associated with COVID-19 infection, including erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), creatinine phosphokinase (CPK), D-dimer, and liver enzymes [24], were also compared. Notable findings included a statistically significant higher rate of tachypnea (p 0.034), as well as higher D-dimer (2.5 mcg/ml v. 0.9 mcg/ml; p 0.017) and LDH levels (410 units/L v. 295.5 units/L; p 0.044) in controls compared to cases (Table 3). Analysis of other secondary outcome variables failed to show any statistically significant differences between the cases and controls.
| - | - | All | Cases | Control | P Value |
| Sex | Female | 84 (81.6%) | 37 (92.5%) | 47 (74.6%) | 0.022 |
| Male | 19 (18.4%) | 3 (7.5%) | 16 (25.4%) | ||
| Age | Mean (SD) | 60.2 (15.9) | 53.3 (15.9) | 64.6 (14.3) | < 0.001 |
| SLE. RA | RA | 70 (68%) | 17 (42.5%) | 53 (84.1%) | < 0.001 |
| SLE | 32 (31.1%) | 22 (55%) | 10 (15.9%) | ||
| SLE & RA | 1 (1%) | 1 (2.5%) | 0 (0%) | ||
| Race | African American | 58 (58%) | 18 (45%) | 40 (66.7%) | 0.029 |
| Other | 22 (22%) | 14 (35%) | 8 (13.3%) | ||
| White | 20 (20%) | 8 (20%) | 12 (20%) |
| All | Cases | Control | P Value | ||
| Smoker | Yes | 2 (1.9%) | 1 (2.5%) | 1 (1.6%) | 1 |
| - | No | 101 (98.1%) | 39 (97.5%) | 62 (98.4%) | - |
| Asthma | Yes | 12 (11.7%) | 6 (15%) | 6 (9.5%) | 0.53 |
| - | No | 91 (88.3%) | 34 (85%) | 57 (90.5%) | - |
| HTN | Yes | 56 (54.4%) | 17 (42.5%) | 39 (61.9%) | 0.054 |
| - | No | 47 (45.6%) | 23 (57.5%) | 24 (38.1%) | - |
| Hyperlipidemia | Yes | 6 (5.8%) | 4 (10%) | 2 (3.2%) | 0.204 |
| - | No | 97 (94.2%) | 36 (90%) | 61 (96.8%) | - |
| COPD | Yes | 5 (4.9%) | 1 (2.5%) | 4 (6.3%) | 0.646 |
| - | No | 98 (95.1%) | 39 (97.5%) | 59 (93.7%) | - |
| CKD | Yes | 6 (5.8%) | 2 (5%) | 4 (6.3%) | 1 |
| - | No | 97 (94.2%) | 38 (95%) | 59 (93.7%) | - |
| ESRD | Yes | 5 (4.9%) | 2 (5%) | 3 (4.8%) | 1 |
| - | No | 98 (95.1%) | 38 (95%) | 60 (95.2%) | - |
| CHF | Yes | 10 (9.7%) | 1 (2.5%) | 9 (14.3%) | 0.084 |
| - | No | 93 (90.3%) | 39 (97.5%) | 54 (85.7%) | - |
| Obesity | Yes | 33 (32%) | 13 (32.5%) | 20 (31.7%) | 0.936 |
| - | No | 70 (68%) | 27 (67.5%) | 43 (68.3%) | - |
| Malignancy | Yes | 4 (3.9%) | 3 (7.5%) | 1 (1.6%) | 0.296 |
| - | No | 99 (96.1%) | 37 (92.5%) | 62 (98.4%) | - |
| DM | Yes | 28 (27.2%) | 12 (30%) | 16 (25.4%) | 0.609 |
| - | No | 75 (72.8%) | 28 (70%) | 47 (74.6%) | - |
| GERD | Yes | 5 (4.9%) | 3 (7.5%) | 2 (3.2%) | 0.374 |
| - | No | 98 (95.1%) | 37 (92.5%) | 61 (96.8%) | - |
| Depression | Yes | 3 (2.9%) | 1 (2.5%) | 2 (3.2%) | 1 |
| - | No | 100 (97.1%) | 39 (97.5%) | 61 (96.8%) | - |
| Fibromyalgia | Yes | 3 (2.9%) | 0 (0%) | 3 (4.8%) | 0.28 |
| - | No | 100 (97.1%) | 40 (100%) | 60 (95.2%) | - |
| Myelodysplastic syndrome | Yes | 1 (1%) | 0 (0%) | 1 (1.6%) | 1 |
| - | No | 102 (99%) | 40 (100%) | 62 (98.4%) | - |
| DVT | Yes | 4 (3.9%) | 2 (5%) | 2 (3.2%) | 0.641 |
| - | No | 99 (96.1%) | 38 (95%) | 61 (96.8%) | - |
| Eczema | Yes | 1 (1%) | 0 (0%) | 1 (1.6%) | 1 |
| - | No | 102 (99%) | 40 (100%) | 62 (98.4%) | - |
| RLD | Yes | 1 (1%) | 0 (0%) | 1 (1.6%) | 1 |
| - | No | 102 (99%) | 40 (100%) | 62 (98.4%) | - |
| CVA | Yes | 2 (1.9%) | 0 (0%) | 2 (3.2%) | 0.52 |
| - | No | 101 (98.1%) | 40 (100%) | 61 (96.8%) | - |
| Hypothyroidism | Yes | 7 (6.8%) | 3 (7.5%) | 4 (6.3%) | 1 |
| - | No | 96 (93.2%) | 37 (92.5%) | 59 (93.7%) | - |
| Sicca | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| - | No | 102 (99%) | 39 (97.5%) | 63 (100%) | - |
| IBS | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| - | No | 102 (99%) | 39 (97.5%) | 63 (100%) | - |
| CAD | Yes | 5 (4.9%) | 0 (0%) | 5 (7.9%) | 0.154 |
| - | No | 98 (95.1%) | 40 (100%) | 58 (92.1%) | - |
| Secondary Adrenal insufficiency | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| - | No | 102 (99%) | 39 (97.5%) | 63 (100%) | - |
| Hyperparathyroidism | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| No | 102 (99%) | 39 (97.5%) | 63 (100%) | - | |
| Pregnancy | Yes | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| No | 103 (100%) | 40 (100%) | 63 (100%) | - | |
| Lupus Nephritis | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| No | 102 (99%) | 39 (97.5%) | 63 (100%) | - | |
| Congenital Heart Block | Yes | 1 (1%) | 1 (2.5%) | 0 (0%) | 0.388 |
| - | No | 102 (99%) | 39 (97.5%) | 63 (100%) | - |
| Interstitial lung disease | Yes | 2 (1.9%) | 0 (0%) | 2 (3.2%) | 0.52 |
| No | 101 (98.1%) | 40 (100%) | 61 (96.8%) | - | |
| Seborrheic dermatitis | Yes | 1 (1%) | 0 (0%) | 1 (1.6%) | 1 |
| - | No | 102 (99%) | 40 (100%) | 62 (98.4%) | - |
| - | - | All | Cases | Control | P Value |
| Diabetic Medications | Yes | 24 (23.3%) | 8 (20%) | 16 (25.4%) | 0.528 |
| - | No | 79 (76.7%) | 32 (80%) | 47 (74.6%) | - |
| Beta blockers | Yes | 27 (26.2%) | 9 (22.5%) | 18 (28.6%) | 0.495 |
| No | 76 (73.8%) | 31 (77.5%) | 45 (71.4%) | - | |
| ACE ARB | Yes | 35 (34.3%) | 11 (28.2%) | 24 (38.1%) | 0.307 |
| - | No | 67 (65.7%) | 28 (71.8%) | 39 (61.9%) | - |
| Colchicine | Yes | 3 (2.9%) | 3 (7.5%) | 0 (0%) | 0.056 |
| - | No | 100 (97.1%) | 37 (92.5%) | 63 (100%) | - |
| Belimumab | Yes | 4 (3.9%) | 4 (10%) | 0 (0%) | 0.021 |
| No | 99 (96.1%) | 36 (90%) | 63 (100%) | - | |
| Steroids | Yes | 22 (21.4%) | 11 (27.5%) | 11 (17.5%) | 0.226 |
| - | No | 81 (78.6%) | 29 (72.5%) | 52 (82.5%) | - |
| Etanercept | Yes | 5 (4.9%) | 2 (5%) | 3 (4.8%) | 1 |
| - | No | 98 (95.1%) | 38 (95%) | 60 (95.2%) | - |
| Methotrexate | Yes | 23 (22.3%) | 2 (5%) | 21 (33.3%) | < 0.001 |
| No | 80 (77.7%) | 38 (95%) | 42 (66.7%) | - | |
| Sulfasalazine | Yes | 3 (2.9%) | 2 (5%) | 1 (1.6%) | 0.558 |
| - | No | 100 (97.1%) | 38 (95%) | 62 (98.4%) | - |
4. DISCUSSION
Chloroquine derivatives have been successfully used as anti-malarials as well as in the management of RA and SLE. Their proposed yet controversial, in vitro antiviral properties sparked a global debate during the COVID-19 pandemic, with several early studies claiming therapeutic and prophylactic properties of these medications, specifically CQ and HCQ, against SARS-CoV-2 [11-14]. These studies were mostly small, non-randomized, and lacked strong methodology. Nevertheless, they gained media attention in the light of a desperate search for treatment and a politicized atmosphere of the COVID-19 pandemic. Several scientific and medical societies opposed such assertions and voiced concern and disagreement towards such claims [25].
While the efficacy of CQ and HCQ was suggested and significantly promoted during the early days of the SARS-CoV-2 pandemic, the in vivo efficacy of these medications were critically questioned, and subsequent studies failed to prove the efficacy of these medications as prophylactic and therapeutic agents [16-22], especially in populations with underlying autoimmune disorders [26-28]. Furthermore, antimalarials, when combined with azithromycin, were associated with adverse cardiovascular outcomes [29-34]. In light of this conflicting data and the significant side effects, the HCQ arm in the SOLIDARITY [35] trial by World Health Organization (WHO) was suspended due to safety concerns raised by Mehra et al. [36]. However, this study was later retracted due to concerns for suspicious unvalidated data [37]. This was followed by suspension of the HCQ arm in the RECOVERY trial [38] due to lack of efficacy, and subsequently by the WHO and National Institute of Health [25].
In our observational study, the number of cases was about two-thirds of controls, and they tended to be younger, although the selection process was completely randomized based on the presence of COVID-19 infection in patients with RA and SLE and taking HCQ at baseline. Despite the average age discrepancy between the two, both cases and controls demonstrated a similar demographic distribution and prevalence of medical comorbidities; therefore, we believe that the possibility of biased selection is very low. Our study failed to show any statistically significant differences in the primary outcomes, including hospitalization, mechanical ventilation, and death, between the cases and controls (Table 2). Among secondary outcomes, tachypnea, D-Dimer, and LDH were found to have significant differences between the cases and controls (Table 3), with higher rates or levels more likely to be associated with those not taking HCQ at baseline (controls). The lower levels of LDH and D-Dimer in cases, which are considered nonspecific markers of inflammation, can be explained by the anti-inflammatory properties of HCQ [4], which have been known and described. Theoretically, less inflammation in the body and specifically less inflammation of the pulmonary system may also explain a lower rate of tachypnea in individuals taking HCQ at baseline (cases). However, limitations to such findings are the multifactorial nature of tachypnea (e.g., pain, fever, anxiety, anemia, infection, etc.) and the examiner-dependent nature of this parameter. Furthermore, the significance of the elevated respiratory rate and elevated inflammatory markers in those not taking HCQ remains unclear, specifically given the lack of differences in the primary outcomes, including mechanical ventilation, hospital length of stay, and death. Cases were more likely to be on belimumab with a possible more potent suppression of the immune system at baseline; however, it is unclear if it directly affected the primary and secondary outcomes of COVID-19 in patients in our study, considering our small sample size. Although methotrexate’s anti-inflammatory properties could have potentially played a role and mask the disease severity and potentially affect primary outcomes, we believe that is less likely as the anti-inflammatory markers were higher in controls compared to cases, indicating a higher inflammatory state in the controls (who were more likely to be on methotrexate) compared to cases.
Several reports have been published on outcomes of COVID-19 in patients with SLE, each failing to demonstrate evidence of a prophylactic or therapeutic role for HCQ [16-22], which is similar to the findings in our study. The report from the COVID-19 Global Rheumatology Alliance by Gianfrancesco et al. [26] highlighted the incidence of COVID-19 in individuals with rheumatic disease, including RA and SLE, among others refuting prior claims that noted an absence of COVID-19 in individuals with the underlying rheumatologic disease taking HCQ [39]. Konig and colleagues subsequently published a report on 80 SLE patients infected with SARS-CoV-2 and demonstrated similar rates of COVID-19 infection in HCQ users and non-users [27]. A French case series reported on 17 patients with SLE infected with SARS-CoV-2, of which 76% developed pneumonia and more highlighted an increased probability of severe COVID-19 pneumonia especially in those with underlying comorbidities, such as chronic kidney disease and obesity. All patients in this study were taking HCQ for at least 6 months, with 65% taking HCQ at 400 mg daily dose with a median HCQ blood concentration of 648 ng/mL. The majority of patients (71%) were also taking maintenance prednisone (no more than 10 mg daily). Nevertheless, 82% were hospitalized with 41% admitted to intensive care unit, and 35% requiring mechanical ventilation [28]. Taken together, these reports highlighted lack of evidence for prophylactic and therapeutic properties of HCQ.
One possible explanation for the lack of efficacy of HCQ, specifically in those with underlying RA or SLE, may relate to epigenetic modifications, with resultant effects on gene transcription and protein translation. Individuals with RA and SLE have demonstrated significantly altered methylation patterns in the promoter regions of multiple genes. Intriguingly, individuals with SLE demonstrated hypomethylation of ACE2, the functional receptor for SARS-CoV-2 [40], whereas individuals with RA demonstrated hypomethylation of IL6 [41], a protein intricately involved in the “cytokine storm” of SARS-CoV-2, with concomitant elevations of both proteins, respectively, a feature that may be further abrogated by the severity of the underlying SLE or RA. A systematic review and meta-analysis revealed an increased risk of COVID-19 in patients with underlying autoimmune diseases and that glucocorticoid use was significantly associated with a higher risk of infection [42]. This increased risk of infection is hypothesized to be related to the suppressed and partially dysregulated immune system in those with underlying autoimmune/ rheumatic disease, their concomitant epigenetic modifications and elevated inflammatory state, and the concomitant use of immunosuppressants, all of which may impair the normal immune response. It is also noteworthy that regular steroid use in patients without respiratory failure, as described in the RECOVERY trial, was not beneficial and possibly associated with adverse outcomes [38]. Our study, however, failed to demonstrate such an association, specifically with respect to steroid use, though the sample size and frequency of steroid use were substantially low (Table 1). Similar to the increasing number of reports demonstrating lack of efficacy of HCQ in preventing or treating COVID-19, a recent systematic review demonstrated no short-term mortality benefit in patients who received HCQ monotherapy and increased mortality in those on combination therapy with azithromycin [43].
CONCLUSION
While the potential prophylactic and therapeutic properties of CQ and HCQ were originally touted by several studies during the early months of the COVID-19 pandemic, subsequent studies including randomized clinical trials and meta-analyses failed to prove such therapeutic potential. Our study, while limited due to its retrospective and observational nature and fairly small sample size, supports emerging data that HCQ at regular doses used in the management of patients with RA or SLE has no prophylactic or therapeutic benefit. Considering the underlying predisposition for inflammation and epigenetic modifications in RA and SLE, as well as increased vulnerability to infection, our study revealed that the prevalence of infection with SARS-CoV-2 was similar in RA and SLE patients with no difference with respect to baseline use of HCQ. Although tachypnea and elevated levels of LDH and D-Dimer were statistically more frequent in controls compared to cases, no difference was observed in primary outcomes such as mechanical ventilation and death among the two groups.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was performed based on the MedStar IRB approval however no ethics committee was required as study was only performed by chart review and names of patients were blinded for confidentiality reasons.
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author [A.M] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGMENTS
Declared none.


