All published articles of this journal are available on ScienceDirect.
Effect of Low-Dose Corticosteroid Use on HBV Reactivation in HBsAg-positive Rheumatoid Arthritis Patients
Abstract
Background:
It is well known that the use of corticosteroids (CS) results in increased viral replication and elevated alanine aminotransferase in hepatitis B virus (HBV) patients. However, only a few studies have investigated the effect of low-dose CS on HBV reactivation. In addition, there are few studies on the effects of synthetic disease-modifying anti-rheumatic drugs on HBV reactivation.
Objective:
We investigated the reactivation of HBV in rheumatoid arthritis (RA) patients treated with long-term low-dose corticosteroids. In addition, we analyzed factors affecting HBV reactivation, including disease-modifying anti-rheumatic drugs.
Methods:
We retrospectively reviewed medical records and analyzed the incidence of HBV reactivation in RA patients who were hepatitis B surface antigen (HBsAg) positive and who were receiving ≤10 mg of prednisolone over 4 weeks. Logistic regression analysis was performed to investigate the factors that increase the risk of HBV reactivation.
Results:
A total of 141 patients were included in the study, out of which 24 (17.0%) patients had HBV reactivation. The administration of low-dose corticosteroids did not affect HBV reactivation in HBsAg-positive RA patients (odds ratio: 0.807, 95% confidence interval: 0.143–4.546, p = 0.808), nor did the duration of corticosteroid administration, average daily corticosteroid dose, and cumulative corticosteroid dose. Administration of leflunomide was found to significantly increase the risk of HBV reactivation (odds ratio: 3.851, 95% confidence interval: 1.026–14.459, p = 0.046).
Conclusion:
The administration of low-dose corticosteroids did not affect the rate of HBV reactivation, suggesting that it can be used safely. Leflunomide may increase the risk of HBV reactivation; therefore, HBV patients should be carefully monitored when receiving this drug.
1. INTRODUCTION
The use of high-dose corticosteroids (CS) in patients with hepatitis B leads to increased replication of the virus and elevated serum alanine aminotransferase concentration [1]. Hepatitis B Virus (HBV) has a transcription regulatory element (glucocorticoid response element) that is activated by CS [2]. This leads to increased viral proliferation when CS are used. In addition, cytotoxic T cells are inhibited by CS, and this promotes viral multiplication [3]. It has previously been reported that when high doses of corticosteroids (prednisolone ≥ 20 mg) were used for more than 4 weeks, the rate of HBV reactivation after steroid withdrawal was 30–67%, with some cases progressing to hepatic failure [4-8]. Therefore, many guidelines recommend that antiviral prophylaxis should be used if 20 mg or more of prednisolone is used for longer than 4 weeks [3, 9, 10]. However, the rate of HBV reactivation in patients receiving low doses of CS (prednisolone < 10 mg/day) is poorly established. There have been some reports of hepatitis aggravation and progression to hepatic failure after low-dose CS administration [11, 12]. However, hepatitis B can be exacerbated by spontaneous viral reactivation [9]. Hence it is difficult to conclude that the cases of hepatitis aggravation and progression were caused by low doses of CS. The American Gastroenterological Association Institute guideline, published in 2015, recommends prophylactic antiviral treatment when CS of less than 10 mg/day are given for more than 4 weeks to patients that are hepatitis B surface antigen (HBsAg) positive or HBsAg negative/hepatitis B core (HBc) antibody-positive [13]. However, due to a lack of evidence, the recommended level of antiviral treatment is low. Hence, we investigated the reactivation of HBV when long-term low-dose corticosteroids were administered to patients with rheumatoid arthritis (RA) who also have hepatitis B. Although many drugs have been developed and are newly used for RA treatment, we still recommend synthetic disease modifying anti-rheumatic drugs (sDMARDs) as primary drugs and they are widely used. According to the current treatment guidelines, sDMARDs are considered to be relatively safe in HBV reactivation compared to biologic disease modifying anti-rheumatic drugs (bDMARDs) in RA patients with HBV, but there are few studies supporting this and the level of evidence is low [10]. Therefore, we simultaneously analyzed factors affecting HBV reactivation including sDMARDs.
2. MATERIALS AND METHODS
This hospital-based observational study was approved by the ethical committee of the institutional review board of Jeju University Hospital (approval number 2013-02-009). All experiments were performed in accordance with the declaration of Helsinki, and the need for written informed consent was waived by the ethics committee.
2.1. Study Design and Patient Selection
We retrospectively reviewed the available data from patients with RA who had the HBsAg in four university hospitals between February 1996 and June 2017. The records from a total of 2437 patients with RA were reviewed; of these, 141 patients were found to be HBsAg positive via the screening test performed at the time of RA diagnosis. We investigated the incidence of HBV reactivation in patients with RA who were HBsAg positive and who were receiving <10 mg of prednisolone or equivalent doses of other steroids for over 4 weeks, and in HBsAg-positive patients with RA who did not receive steroids. Patients who received antiviral prophylaxis were excluded from this study. All patients in this study fulfilled the American College of Rheumatology 1987 revised criteria or 2010 American College of Rheumatology/European League Against Rheumatism collaborative initiative classification criteria for RA.
2.2. Drugs and Definition
The sDMARDs used in this study were methotrexate (MTX), leflunomide (LEF), hydroxychloroquine (HCQ), sulfasalazine (SSZ), tacrolimus, and cyclosporine. The biologic disease-modifying anti-rheumatic drugs (bDMARDs) included tumor necrosis factor inhibitor (TNFi), tocilizumab, and abatacept. Drug exposure was considered when a patient took CS, sDMARDs, or bDMARDs for > 4 weeks.
We defined HBV reactivation as >10 times increase in HBV DNA level or reappearance of HBV DNA in the serum, as in previous studies [14, 15]. Low-dose CS was defined as < 10 mg of prednisolone (PD) or its equivalent.
2.3. Statistical Analysis
Data are presented as mean ± standard deviation for continuous variables or as frequencies for categorical variables. The significance of differences between groups was determined by using the t-test and chi-squared test. Logistic regression analysis was performed to investigate the factors that increase the risk of HBV reactivation. All statistical analyses were performed using SPSS version 20.0 (for Windows; SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Demographic and Clinical Characteristics of the Study Population
Among 141 patients who were positive for HBsAg, 103 (73%) were females, and the mean age at diagnosis was 50.5 (17-78) years (Table 1). The average disease duration for RA patients was 9.2 (0.1-33.5) years. Some patients had underlying diseases such as diabetes mellitus (13) (9.2%) and hypertension (22) (15.6%). Five (3.5%) had a history of pulmonary tuberculosis, and 3 (2.1%) had a history of malignancy. None of the patients with malignancies were receiving chemotherapy. The mean baseline levels of aspartate aminotransferase and alanine aminotransferase were 28.2 U/L and 26.9 U/L, respectively.
In total, 128 patients (90.8%) were taking CS and the mean daily CS dose (converted to PD dose) was 4.3 (0.23-9.7) mg. Other immunosuppressive drugs taken by patients were MTX (60, 42.6%), LEF (12, 8.5%), SSZ (70, 49.6%), HCQ (100, 70.9%), tacrolimus (6, 4.3%), and cyclosporine (3, 2.1%). A total of 7 (5.0%) patients received TNFi.
3.2. Incidence and Baseline Characteristics of Hepatitis B virus Reactivation
HBV reactivation occurred in 24 (17.0%) of the included patients. The patients were divided into a CS administration group and a non-CS administration group (Table 1). There was no significant difference in age (50.3 vs. 55.5, p = 0.130) or gender (female, 71.1% vs. 92.3%, p = 0.186) between the two groups. There was no substantial difference between the two groups in terms of the underlying disease. Further, the HBV profile did not differ between the two groups. There was no significant difference in disease-modifying anti-rheumatic drugs (DMARDs) use for any of the following drugs: MTX (43.8% vs. 30.8%, p = 0.558), LEF (8.6% vs. 7.7%, p = 1.000), SSZ (51.6% vs. 30.8%, p = 0.244), HCQ (71.9% vs. 61.5%, p = 0.523), and TNF-α inhibitor (5.5% vs. 0%, p = 1.000). The mean duration from commencement of CS administration to HBV reactivation was 46.4 (1.2-215.1) months. The number of patients with HBV reactivation after CS discontinuation was 2 (1.4%), and the meantime to HBV reactivation after CS discontinuation was 22.2 (15.2-35.4) months. Seven patients received TNFi.
When the patients were divided into HBV-reactivated and HBV-non-reactivated groups (Table 2), the mean age was 50.0 vs. 50.6 (p = 0.829). There was no difference in baseline characteristics between the two groups. The proportion of patients receiving CS did not differ considerably between the two groups (91.7 vs. 90.6, p = 1.000). The proportion of patients receiving MTX, cyclosporine, tacrolimus, and TNFi did not differ, but there were more patients receiving LEF (20.8% vs. 6.0%, p = 0.033) and HCQ (87.5% vs. 67.5%, p = 0.052) in the group of patients who had HBV reactivation. HBV reactivation occurred in 41.7% of patients receiving LEF and 28.6% of patients receiving TNF- α inhibitor.
The mean duration of CS administration (30.5 months vs. 49.8 months, p = 0.106) and mean CS administration dose (4.1 mg vs. 3.8 mg, p = 0.643) were higher in the group of patients who had HBV reactivation, but the differences were not statistically significant. During low-dose CS administration, more than half of HBV reactivation (54.5%) occurred within a year (Fig. 1). The mean duration between HBV reactivation after RA treatment was 60.8 (1.4-257.7) months.
| - | Total | CS use | CS non-use | P-value | |
|---|---|---|---|---|---|
| - | 141 | 128 (90.8) | 13 (9.2) | - | |
| Sex, female | 103 (73.0) | 91 (71.1) | 12 (92.3) | 0.186 | |
| Age | 50.5 ± 12.3 | 50.0±12.5 | 55.5±8.8 | 0.130 | |
| Comorbidities | |||||
| DM | 13 (9.2) | 13 (10.2) | 0 (0) | 0.610 | |
| HT | 22 (15.6) | 22 (17.2) | 0 (0) | 0.221 | |
| Pul. Tb | 5 (3.5) | 4 (3.1) | 1 (7.7) | 0.388 | |
| malignancy | 3 (2.1) | 2 (1.6) | 1 (7.7) | 0.254 | |
| Alcohol | 6 (4.3) | 6 (4.7) | 0 (0) | 1.000 | |
| HBV profile | |||||
| HBsAb | 3 (2.5) | 3 (2.5) | 0 (0) | 1.000 | |
| HBeAb | 85 (60.3) | 75 (78.9) | 10 (100) | 0.203 | |
| HbeAg | 17 (12.1) | 17 (17.9) | 0 (0) | 0.360 | |
| HBcAb | 77 (54.6) | 67 (91.8) | 10 (100) | 1.000 | |
| DNA copy (log, IU/mL) | 2.35±2.54 | 2.39±2.60 | 2.07±1.96 | 0.666 | |
| HBV reactivation | 24 (17.0) | 22 (17.2) | 2 (15.4) | 1.000 | |
| AST | 28.2±34.3 | 28.6±37.5 | 23.8±5.8 | 0.673 | |
| ALT | 26.9±36.0 | 27.6±37.5 | 19.8±12.5 | 0.458 | |
| DMARDs | |||||
| MTX | 60 (42.6) | 56 (43.8) | 4 (30.8) | 0.558 | |
| LEF | 12 (8.5) | 11 (8.6) | 1 (7.7) | 1.000 | |
| SSZ | 70 (49.6) | 66 (51.6) | 4 (30.8) | 0.244 | |
| HCQ | 100 (70.9) | 92 (71.9) | 8 (61.5) | 0.523 | |
| TC | 6 (4.3) | 6 (4.7) | 0 (0) | 1.000 | |
| Cys | 3 (2.1) | 3 (2.3) | 0 (0) | 1.000 | |
| TNFi | 7 (5.0) | 7 (5.5) | 0 (0) | 1.000 | |
The numbers in brackets indicate a percentage.
| - | HBV reactivation | HBV non-reactivation | P-value |
|---|---|---|---|
| - | 24 (17.0) | 117 (83.0) | - |
| Sex, female | 17 (70.8) | 86 (73.5) | 0.788 |
| Age | 50.0±13.6 | 50.6±12.1 | 0.829 |
| HBV profile | |||
| HBsAb | 0 (0) | 3 (2.7) | 1.000 |
| HbeAg | 2 (13.3) | 15 (16.7) | 1.000 |
| HBeAb | 12 (85.7) | 73 (80.2) | 1.000 |
| HBcAb | 13 (100) | 64 (91.4) | 0.583 |
| HBV DNA copy (log, IU/mL) | 1.91±2.6 | 2.45±2.5 | 0.347 |
| AST | 37.3±62.0 | 26.3±25.2 | 0.402 |
| ALT | 41.3±77.8 | 23.9±17.7 | 0.286 |
| DMARDs | |||
| MTX | 11 (45.8) | 49 (41.9) | 0.721 |
| LEF | 5 (20.8) | 7 (6.0) | 0.033* |
| SSZ | 15 (62.5) | 55 (47.0) | 0.167 |
| HCQ | 21 (87.5) | 79 (67.5) | 0.052 |
| TC | 2 (8.3) | 4 (3.4) | 0.270 |
| Cys | 0 (0) | 3 (2.6) | 1.000 |
| TNFi | 2 (8.3) | 5 (4.3) | 0.340 |
| Corticosteroid | |||
| CS use | 22 (91.7) | 106 (90.6) | 1.000 |
| CS duration (month) | 30.5±45.6 | 49.8±54.4 | 0.106 |
| Mean CS dose (converted to PD dose) | 4.1±2.1 | 3.8±2.2 | 0.643 |
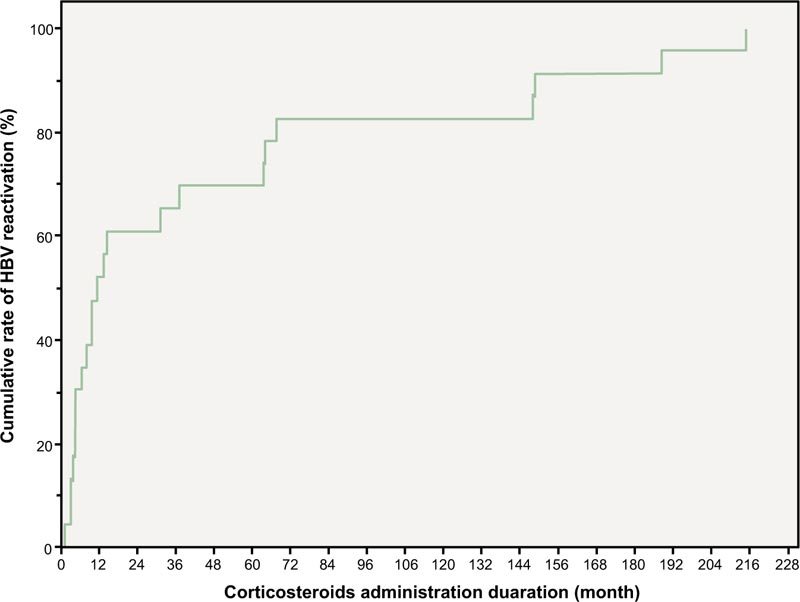
More than half (54.5%) of the HBsAg-positive RA patients receiving low-dose CS showed a tendency to develop HBV reactivation within 1 year after CS administration.
3.3. Factors Associated with Hepatitis B Virus Reactivation
To identify factors affecting HBV reactivation, logistic regression was performed on CS, immunosuppressive drugs, and other known risk factors of HBV reactivation (Table 3). Univariate logistic regression analysis showed that CS administration, mean CS administration dose, and cumulative CS administration dose did not affect HBV reactivation. However, LEF administration was found to significantly increase the risk of HBV reactivation (odds ratio 4.135, 95% CI 1.189–14.386, p = 0.026), and HCQ administration showed a tendency to increase the risk of HBV reactivation (odds ratio 3.367, 95% CI 0.945–11.991, p = 0.061). In the multiple logistic regression analysis, only LEF administration was found to significantly increase the risk of HBV reactivation (odds ratio 3.851, 95% CI 1.026–14.459, p = 0.046).
| - | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Age | 0.995 | 0.956-1.036 | 0.814 |
| Sex | 0.764 | 0.268-2.177 | 0.615 |
| HBV DNA copy (log, IU/mL) | 0.948 | 0.779-1.154 | 0.596 |
| CS use | 0.807 | 0.143-4.546 | 0.808 |
| LEF | 3.851 | 1.026-14.459 | 0.046* |
| HCQ | 2.986 | 0.788-11.305 | 0.107 |
| SSZ | 1.451 | 0.552-3.817 | 0.450 |
| TNFi | 1.329 | 0.204-8.642 | 0.766 |
p < 0.05 considered significant and marked with *
4. DISCUSSION
Low-dose CS (< 10 mg prednisone or its equivalent) therapies administered for over 4 weeks may increase the risk of viral reactivation by up to 10% in HBsAg-positive individuals [16]. About 10–20% of HBV inactive carriers progress to cirrhosis due to recurrent reactivation and inactivation [9, 17]. In general, HBV reactivation is known to occur much more frequently (up to eight times) in HBsAg-positive patients than in HBsAg-negative / anti-HBc-positive patients [16, 18]. Pattullo et al. reported that HBV reactivation in RA patients occurred in 12.3% of HBsAg-positive patients and 3–5% of HBsAg-negative / anti-HBc-positive patients [18]. Chen et al. showed that the combination of immunosuppressive drugs increases the risk of HBV reactivation [19]. In our study, HBV reactivation occurred in 17.1% of patients taking low-dose CS. This is a higher reactivation rate compared to previous studies [18, 20], which may be because all study participants were HBsAg positive and were taking immunosuppressive drugs in addition to low-dose CS. Herein, low-dose CS did not appear to increase the risk of HBV reactivation in HBsAg-positive patients with RA. Therefore, antiviral prophylaxis may not be required when low-dose CS are administered in HBsAg-positive patients with RA.
HBV reactivation can occur within the first 2 weeks of commencing chemotherapy or within 1 year of withdrawing immunosuppressive therapy [16]. In this study, none of the patients taking low-dose CS had HBV reactivation within a month. However, more than half (54.5%) of the cases of HBV reactivation occurred within a year. Therefore, even if the risk of HBV reactivation is low when taking low-dose CS, it is necessary to carefully monitor HBV reactivation for 1 year.
We analyzed the factors affecting HBV reactivation and found that the use of CS did not affect HBV reactivation. In contrast, the use of LEF did increase the risk of HBV reactivation significantly. Our findings are consistent with those of Xu et al., who showed a 30% increased risk of HBV reactivation in patients taking 10 mg of LEF [21]. The American Gastroenterological Association Institute guideline classifies MTX as a low-risk drug and states that prophylactic antiviral therapy should not be used routinely, but there is no mention of HBV reactivation risks associated with the administration of LEF and other sDMARDs. Although it is not yet possible to form a definite conclusion, LEF administration seems to increase the risk of HBV reactivation based on the results of previous studies and our study. Therefore, it is necessary to monitor closely for HBV reactivation when LEF is administered to patients with RA. A well-designed study of HBV reactivation secondary to LEF is needed to verify the findings of this study.
Biologic agents, such as TNFi, have been developed and are widely used. These drugs have been classified as moderate-risk drugs with regard to HBV reactivation. In the current study, 7 patients received TNFi, 2 (28.6%) of whom developed HBV reactivation; and both cases occurred during the administration of adalimumab. This reactivation rate is similar to that of previous studies on HBV reactivation during TNFi administration [22, 23]. Although the administration of TNFi did not increase the risk of HBV reactivation, the number of patients who were administrated TNFi was too small to determine statistical significance.
Several limitations of this study should be considered. The main limitations of this study were the cross-sectional, retrospective, observational design, and the laboratory data used were obtained by reviewing medical records. Secondly, the number of patients not receiving CS was small. Third, only the effect of DMARD administration on HBV reactivation was analyzed. The effect on HBV reactivation according to the dose of DMARDs was not analyzed.
CONCLUSION
Our results show that low-dose CS administration had no additional effect on HBV reactivation in HBsAg-positive patients with RA who are taking DMARDs, suggesting that it is safe to use such drugs in this patient population. However, the combination of low-dose CS with immunosuppressants such as LEF may increase the risk of HBV reactivation. In such cases, it may be safer to monitor HBV reactivation more carefully, including the check of HBV DNA titer. Further prospective studies on the effects of sDMARDs on HBV reactivation are needed.
LIST OF ABBREVIATIONS
| CS | = Corticosteroids |
| HBV | = Hepatitis B Virus |
| HBsAg | = Hepatitis B Surface Antigen |
| HBc | = Hepatitis B Core Antibody |
| RA | = Rheumatoid Arthritis |
| sDMARDs | = Synthetic Disease-Modifying Anti-Rheumatic Drugs |
| MTX | = Methotrexate |
| LEF | = Leflunomide |
| HCQ | = Hydroxychloroquine |
| SSZ | = Sulfasalazine |
| bDMARDs | = Biologic Disease-Modifying Anti-Rheumatic Drugs |
| TNF | = Tumor Necrosis Factor |
| PD | = Prednisolone |
| DMARDs | = Disease Modifying Anti-Rheumatic Drugs |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This hospital-based observational study was approved by the ethical committee of the institutional review board of Jeju National University Hospital (approval number 2013-02-009).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
The datasets generated and/or analyzed during the current study are not publicly available in order to protect the privacy of the study participants. However, they are available from the corresponding author [J.K] on reasonable request.
FUNDING
This work was supported by a research grant from Jeju National University Hospital in 2016.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We thank Sehee Kim for supporting us in reviewing and filling up research data.


