All published articles of this journal are available on ScienceDirect.
Clinical, Radiologic, and Functional Outcomes Following Methotrexate Withdrawal in Etanercept-Treated Patients with Active Early Rheumatoid Arthritis: A Subanalysis of COMET Year 2 by Week 52 DAS28 Status
Abstract
Introduction:
This post-hoc analysis explored Methotrexate (MTX) withdrawal on clinical, radiographic, and functional outcomes in patients with early rheumatoid arthritis who previously received 52 weeks of Etanercept (ETN) + MTX treatment in the COMET study.
Methods:
Response at week 104 was analyzed based on the attainment of remission (28-joint disease activity score [DAS28] <2.6; Boolean); low disease activity (LDA; 2.6 ≤DAS28 ≤3.2); normal Health Assessment Questionnaire-Disability Index (HAQ-DI) score (≤0.5); or radiographic non-progression (change in modified Total Sharp Score ≤0.5).
Results:
Of 208 patients with baseline DAS28 scores at week 52, 105 received ETN + MTX and 103 received ETN over weeks 52-104 (Period 2). At week 104, rates of LDA (70% vs 67%), remission (59% vs 52%), and normal HAQ-DI (63% vs 61%) were similar in both arms; week 52 responders also had a higher response rate at week 104 irrespective of treatment during Period 2. Overall rates of radiographic non-progression were higher for ETN + MTX (90%) vs ETN (74%) at week 104; week 52 non-responders in the Period 2 ETN + MTX arm had a 21-27% higher rate vs ETN, while the treatment difference was 11-12% for week 52 responders.
Conclusion:
The data suggest that for responders to ETN + MTX at week 52, MTX may be safely withdrawn. For non-responders where de-escalation would not be considered, the continuation of the combination is advisable. Radiological outcome was numerically worse, but of uncertain clinical significance.
1. INTRODUCTION
Etanercept (ETN) is a fully human, soluble recombinant Tumor Necrosis Factor (TNF) receptor fusion protein indicated as monotherapy or in combination with methotrexate (MTX) for moderate-to-severe Rheumatoid Arthritis (RA) [1]. In clinical trials, treatment with ETN plus MTX nearly halts structural progression and produces clinically relevant responses in patients with RA [2, 3]. For patients treated with ETN or other biological Disease-modifying Antirheumatic Drugs (bDMARDs) in combination with MTX, European League Against Rheuma- tism (EULAR) and American College of Rheumatology (ACR) treatment guidelines advise de-escalation of therapy once the treatment target (e.g. Low Disease Activity [LDA] or remission) has been achieved [4, 5]. Most de-escalation studies have investigated tapering of the bDMARD component of therapy [6]. The Combination of Methotrexate and Etanercept in Early Rheumatoid Arthritis (COMET) trial was the first randomized controlled study to assess withdrawal of MTX from bDMARD combination therapy [7, 8]. This is a clinically relevant question, as side effects are commonly reported with MTX treatment [9].
The COMET trial compared remission rates and radio- graphic progression with MTX monotherapy or ETN plus MTX in patients with early, moderate-to-severe RA [7]. Following treatment with ETN plus MTX, 50% of patients achieved clinical remission (based on 28-joint disease activity score (DAS28)) at 52 weeks and 80% achieved radiographic non-progression [7].
The objective of this post-hoc analysis was to explore the effect of 52 weeks of MTX withdrawal on clinical remission and radiographic non-progression in patients with RA who had previously received ETN plus MTX in the first 52 weeks of COMET.
2. METHODS
2.1. Study Design and Patient Selection
COMET is a 24-month, double-blind, randomized, parall- el-group, multicenter study in outpatients aged 18 years or older with adult-onset, moderate-to-severe RA. Details of the study have been described previously [7].
Briefly, the study was conducted in two periods. In Period 1, participants were randomly assigned to receive ETN (50 mg/ week, administered as two separate subcutaneous injections of 25 mg each on the same day) plus oral MTX (starting dose 7.5 mg/week, titrated to a maximum of 20 mg/week over 8 weeks in patients with tender or swollen joints), or oral MTX plus placebo (administered as subcutaneous injections once a week) for 52 weeks. Stable doses of oral corticosteroids (≤10 mg per day of prednisone or an equivalent agent) or a single non-steroidal anti-inflammatory drug were permitted if they had been started at least 4 weeks before baseline and kept constant throughout the first 24 weeks of the study.
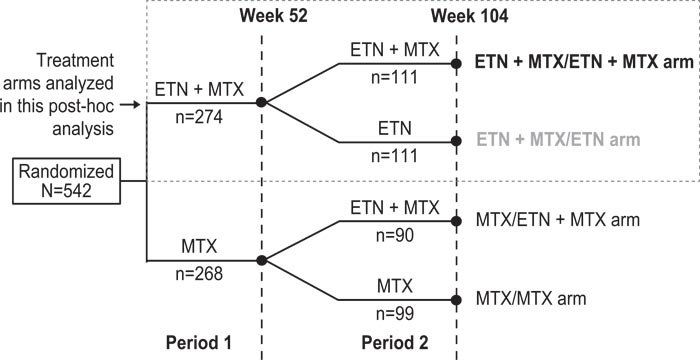
In Period 2 (weeks 52-104), patients were randomized to either continue with the assigned Period 1 treatment, have MTX withdrawn (for those receiving ETN + MTX during Period 1), or have ETN added (for those receiving MTX + placebo during Period 1) (Fig. 1). Randomization for Period 1 and Period 2 treatments was performed at the start of the study, with no re-randomization occurring at the end of Period 1.
This post-hoc analysis focused only on patients who received ETN + MTX in Period 1, and either continued with combination therapy (ETN + MTX then ETN + MTX) or had MTX withdrawn (ETN + MTX then ETN) in Period 2 (Fig. 1).
2.2. Study Outcomes and Data Analysis
The response to therapy at the end of week 104 was analyzed based on the attainment of either: (1) LDA (2.6 ≤DAS28 ≤3.2); (2) remission (DAS28 <2.6); (3) Normal Health Assessment Questionnaire-Disability Index (HAQ-DI) score (≤0.5); or (4) Radiographic non-progression (change from baseline to week 104 in modified Total Sharp Score (mTSS) of ≤0.5).
Patients were stratified based on their clinical response at the end of Period 1 (week 52) into (1) those who achieved or did not achieve DAS28 LDA and (2) those who achieved or did not achieve remission. Demographic and baseline disease characteristics were analyzed according to week 52 DAS28 categories (remission, LDA (excluding remission), non-response). Continuous characteristics were analyzed using an ordered logistic regression model of week 52 DAS28 categories with baseline parameter as a predictor. Categorical characteristics were analyzed using the Cochran-Mantel- Haenszel test of linear association. This was performed for pooled treatment arms.
Odds Ratios (ORs) of achieving selected outcomes at week 104 for patients treated with continued ETN + MTX treatment versus those who had MTX withdrawn were calculated using logistic regression models with treatment, week 52 DAS28 status, and their interaction as predictors of week 104 selected outcomes. For each week 52 DAS28 stratum, response rates were calculated for each week 104 outcome, according to treatment arm.
3. RESULTS
Of 274 patients who received ETN + MTX in Period 1, 208 patients had known baseline DAS28 scores at the start of Period 2 (week 52); for DAS28 at week 52, 56% (117/208) were in remission, 13% (27/208) had LDA, and 31% (64/208) were non-responders. Non-responders were more likely to be female, have higher Period 1 baseline DAS28, painful joints count, fatigue, HAQ-DI score and Clinical Disease Activity Index (CDAI), and higher mTSS compared with patients in remission (Table S1).
Of the 208 patients with known baseline DAS28 scores at week 52, 105 received ETN + MTX and 103 received ETN during Period 2. Patterns in patient demographics and Period 2 baseline (i.e. week 52) disease characteristics across the DAS28 response categories were broadly similar for those receiving ETN + MTX and those receiving ETN in Period 2 (Table 1). Patients who were in remission at week 52 had fewer painful joints and lower DAS28, fatigue, HAQ-DI, and CDAI scores at Period 2 baseline (i.e. week 52) than patients who were week 52 non-responders. In contrast, being female was associated with LDA and non-responder status at week 52 in those who went on to receive ETN + MTX in Period 2, but not in those who went on to receive ETN (Table 1).
At week 104, overall rates of DAS28 LDA (70% vs 67.0%), DAS28 remission (59% vs 52%), and normal HAQ-DI (63% vs 61%) were similar in patients receiving ETN + MTX versus ETN during Period 2 (Fig. 2A-C). Rates of radio- graphic non-progression were higher in patients receiving ETN + MTX (90%) versus ETN (74%) during Period 2 (Fig. 2D).
| - | ETN + MTX/ETN + MTX | ETN + MTX/ETN | ||||
|---|---|---|---|---|---|---|
| Characteristic | Remission (n=54) |
LDA (Excluding Remission) (n=15) |
Non-responder (n=36) | Remission (n=63) |
LDA (Excluding Remission) (n=12) |
Non-responder (n=28) |
| Age [median], years (range) | 54.3 (24.1–79.3) | 64.5 (29.4–82.8) | 51.9 (23.7–70.7) | 55.4 (19.1–72.7) | 60.4 (31.7–70.9) | 56.0 (19.8–79.2) |
| Female, n (%) | 28 (52) | 14 (93) | 33 (92) | 45 (71) | 8 (67) | 24 (86) |
| Disease duration [median], months (range) | 6.0 (3.0–24.0) | 6.0 (3.0–24.0) | 7.0 (3.0–24.0) | 8.0 (3.0–24.0) | 7.0 (3.0–12.0) | 10.0 (3.0–22.0) |
| DAS28 [median] (interquartile range) | 1.8 (1.3–2.1) | 3.0 (2.8–3.1) | 3.9 (3.5–4.1) | 1.9 (1.6–2.2) | 2.9 (2.8–3.0) | 4.0 (3.6–4.7) |
| Painful joints–28 joint count [median] (interquartile range) | 0.0 (0.0–0.0) | 1.0 (0.0–2.0) | 2.0 (1.0–6.0) | 0.0 (0.0–0.0) | 1.0 (1.0–2.5) | 3.0 (2.0–6.5) |
| Fatigue [median] (interquartile range) | 12.0 (3.0–31.0) | 35.0 (12.0–51.0) | 49.0 (25.0–66.0) | 12.0 (3.0–26.0) | 25.0 (5.0–52.5) | 42.5 (21.5–60.0) |
| HAQ-DI, mean (SD) | 0.4 (0.5) | 0.9 (0.7) | 0.9 (0.7) | 0.2 (0.4) | 0.7 (0.7) | 1.2 (0.7) |
| CDAI, median (interquartile range) | 3.0 (2.0–4.0) | 7.0 (6.0–9.0) | 14.5 (8.0–17.0) | 2.0 (1.0–5.0) | 6.0 (5.0–10.5) | 11.0 (8.5–18.5) |
| mTSS, median (interquartile range) | 2.0 (0.5–5.0) | 6.3 (3.5–21.0) | 2.0 (1.0–4.5) | 3.0 (0.5–10.0) | 3.8 (1.5–23.0) | 6.0 (0.5–9.5) |
ETN, etanercept; MTX, methotrexate.
Rates and ORs of achieving DAS28 LDA, DAS28 remi- ssion, and normal HAQ-DI at week 104 were two- to three-times higher (non-significant) and 10 to 15 times higher odds (significant) in week 52 responders (i.e. patients with DAS28 LDA or DAS28 remission at week 52) versus non-responders both in the ETN + MTX and ETN arms. The non-responder arm receiving ETN had the lowest rate of response (Figs. 2A-C and Fig. 3). However, odds of achieving LDA, remission, or normal HAQ-DI at week 104, though higher for week 52 responders, were not significantly higher (i.e. ORs included 1) for ETN + MTX versus ETN, irrespective of DAS28 status at week 52 (Fig. 2).
Similar but more pronounced results were seen for week 104 Boolean remission in week 52 Boolean remission res- ponders versus non-responders, whereby rates for week 104 Boolean remission were roughly four- to six-times higher for week 52 remitters versus non-remitters (Fig. 4). In patients who had not achieved Boolean remission at week 52, 22% of those receiving ETN + MTX in Period 2 went on to achieve Boolean remission at week 104 compared with 11% of those receiving ETN alone. In patients who achieved Boolean remission at week 52, 78% of those receiving ETN + MTX in Period 2 maintained Boolean remission to week 104 compared with 71% of those receiving ETN alone.
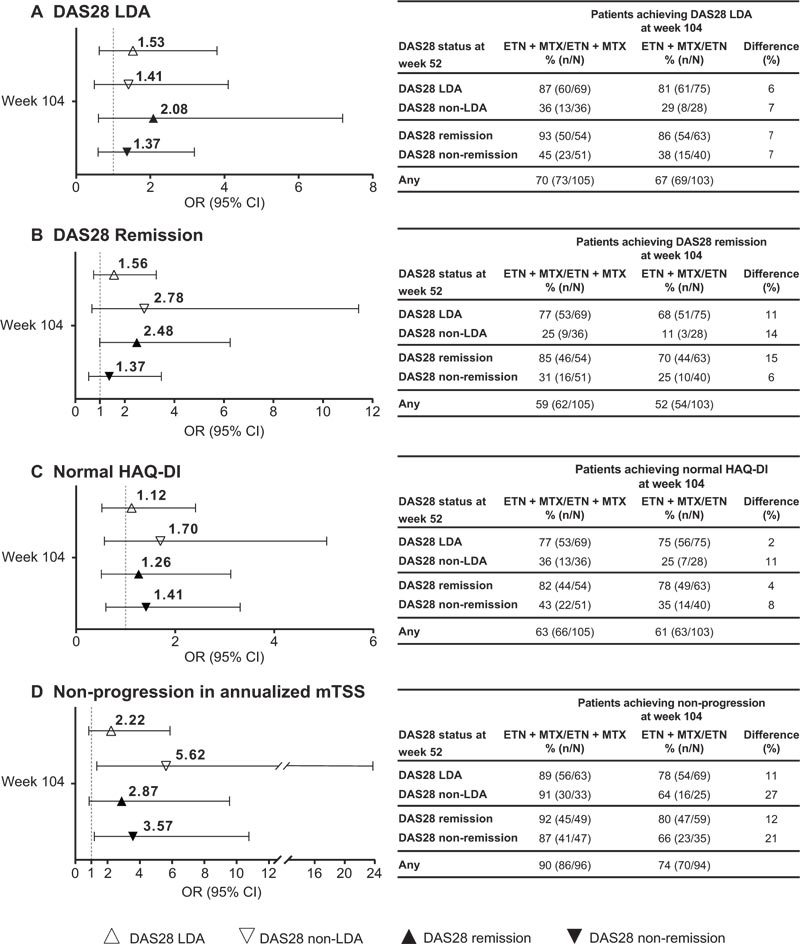
CI, Confidence Interval; DAS28, 28-joint Disease Activity Score; ETN, Etanercept; HAQ-DI, Health Assessment Questionnaire-Disability Index; LDA, Low Disease Activity; mTSS, modified Total Sharp Score; MTX, Methotrexate; OR, Odds Ratio. Bars indicate upper and lower 95% CIs. Difference (%) = difference between treatment arms at week 104. DAS28 LDA = DAS28 ≤3.2. DAS28 remission = DAS28 <2.6. Normal HAQ-DI = HAQ ≤0.5. Non-progression in annualized mTSS = change from year 2 baseline mTSS ≤0.5.
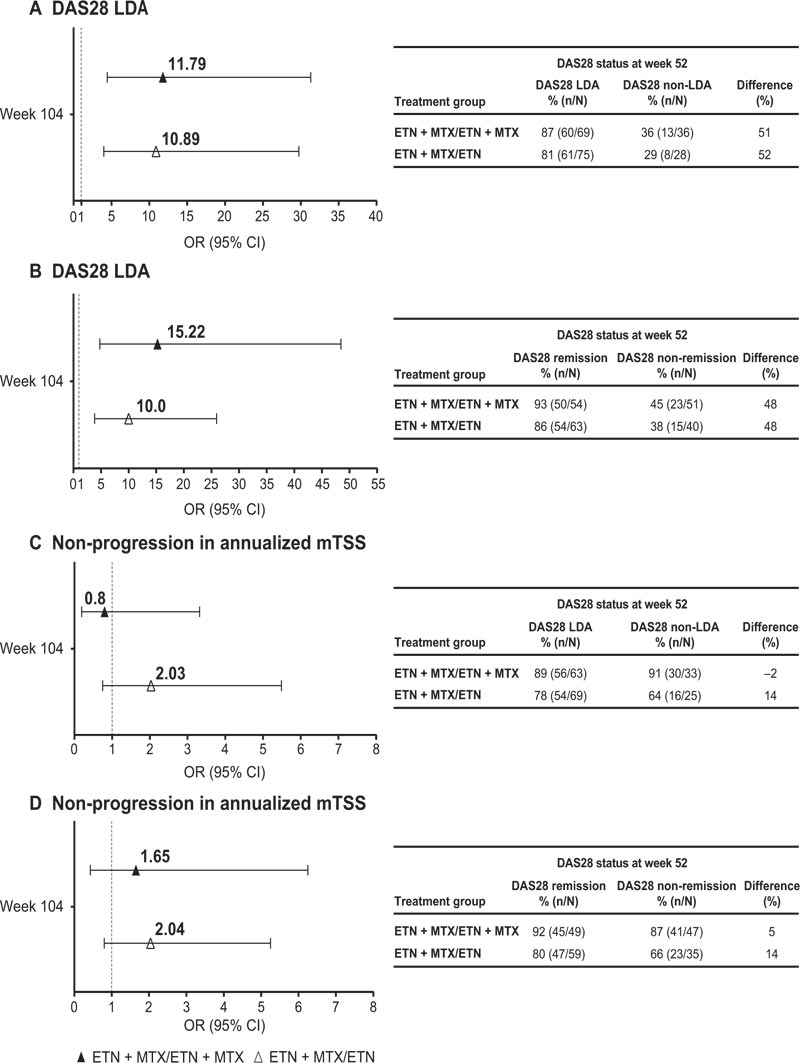
CI, Confidence Interval; DAS28, 28-joint Disease Activity Score; ETN, Etanercept; mTSS, modified Total Sharp Score; MTX, Methotrexate. Bars indicate upper and lower 95% CIs. Difference (%) = difference between treatment week 52 response/non-response groups in achieving radiographic non-progression at week 104. DAS28 LDA = DAS28 ≤3.2. DAS28 remission = DAS28 <2.6.
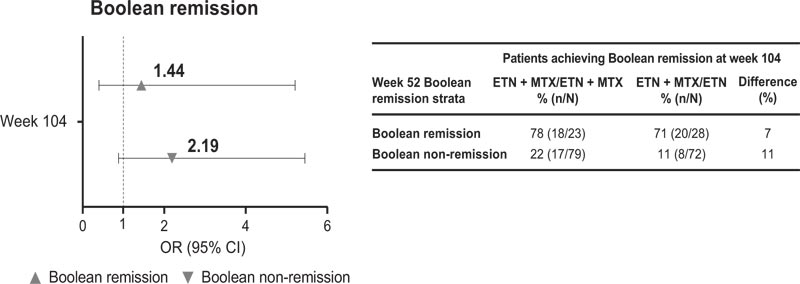
CI, Confidence Interval; ETN, Etanercept; MTX, Methotrexate; OR, Odds Ratio. Bars indicate upper and lower 95% CIs. Difference (%) = difference between treatment arms at week 104. Only patients with baseline and post-baseline values included for on-therapy visits. The OR compares ETN + MTX/ETN + MTX vs ETN + MTX/ETN, obtained from logistic regression models with treatment, week 52 Boolean remission status, and their interaction as predictors. Models are generated separately for each timepoint.
Rates of achieving radiographic non-progression at week 104 in the ETN arm were higher in week 52 responders (78-80%) versus non-responders (64-66%) (Fig. 2D). How- ever, in the ETN + MTX arm, rates of achieving radiographic non-progression at week 104 were similarly high in week 52 responders (89-92%) and non-responders (87-91%) (Fig. 2D). Patients who were non-responders at week 52 had a 21-27% higher rate (3.6 to 5.6 times higher odds) of achieving radiographic non-progression at week 104 if they received ETN + MTX versus ETN alone in Period 2; this difference between treatment arms was 11-12% (OR = 2.2 to 2.9) in those who were week 52 responders (Fig. 2D).
4. DISCUSSION
The COMET trial reported improvements in clinical remission and radiographic non-progression with ETN + MTX at 1 year in patients with early, moderate-to-severe RA [7]. In this post-hoc analysis, to our knowledge the first randomized study of MTX withdrawal from bDMARD combination thera- py, we observed higher rates of response (based on LDA and remission) at 2 years in those patients who were responders to ETN + MTX at 1 year compared with non-responders. This finding was irrespective of whether patients continued to receive ETN + MTX or were de-escalated to ETN during the second year. In patients who were responders at week 52, subsequent discontinuation of MTX for 52 weeks resulted in minimal loss of clinical (based on DAS28 LDA and remission), functional (based on HAQ-DI), and radiological (based on mTSS) efficacy compared with continuing on ETN + MTX. Treatment with MTX is associated with a number of serious side effects, including infections, hematologic effects, lympho- proliferative disorders, nephrotoxicity, and hepatotoxicity, and 20-30% of patients with RA discontinue MTX within their first year of therapy as a result [10-14]. Discontinuation of MTX after achieving LDA or remission may benefit patients by reducing the potential for adverse events, especially in elderly patients who may have renal or hepatic dysfunction or may be more susceptible to dosage errors. This approach also follows EULAR and ACR guidelines for patients with RA treated with DMARDs in combination with MTX, which advise de-escalation of therapy once the treatment target has been achieved [4, 5], thus providing new evidence to support guidelines for MTX tapering. Although the concomitant use of MTX can also help to reduce immunogenicity seen with the use of biologics [15, 16], ETN has a low immunogenicity profile [17], meaning patients do not require this added suppression of immunogenicity afforded by MTX.
In patients who did not achieve LDA or remission after 1 year of ETN + MTX, we observed significantly greater odds of achieving radiographic non-progression if patients continued on ETN + MTX rather than having MTX withdrawn. For these patients, our findings support continuation of combination therapy and withdrawal of MTX as soon as remission is achieved. The findings have clear implications for manage- ment; responders can taper MTX successfully while non-responders need to continue the combination to maintain their level of response.
This study has a number of limitations. This was a post-hoc analysis of COMET, and the original study was not powered to allow firm conclusions to be drawn on the impact of withdrawing MTX based on week-52 DAS28 response status. Furthermore, while MTX withdrawal was considered, there was no assessment of the number of patients who received MTX dose reductions during the study.
CONCLUSION
Notwithstanding these limitations, this post-hoc analysis may help to guide the management of RA. For responders to the combination ETN + MTX (especially those in remission), the data suggest that MTX can be safely withdrawn. For those not achieving LDA, in whom de-escalation would not normally be considered, the data indicate that continuation of the combination is advisable.
LIST OF ABBREVIATIONS
| CDAI | = Clinical Disease Activity Index |
| DAS28 | = 28-joint Disease Activity Score |
| dMARD | = disease-Modifying Antirheumatic Drug |
| ETN | = Etanercept |
| HAQ-DI | = Health Assessment Questionnaire-Disability Index |
| LDA | = Low Disease Activity |
| mTSS | = modified Total Sharp Score |
| MTX | = Methotrexate |
| OR | = Odds Ratio |
| RA | = Rheumatoid Arthritis |
| TNF | = Tumor Necrosis Factor |
AUTHORS' CONTRIBUTIONS
All authors contributed to the writing of this manuscript, the analysis and interpretation of data, and provided critical manuscript revision and approval of final content.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The protocol and its amendments received independent ethics committee or institutional review-board approval and regulatory review and approval before site initiation and recruitment of patients.
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
AVAILABILITY OF DATA AND MATERIALS
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/ clinical-trials/trial-data-and-results for more information), Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices: (1) for indications that have been approved in the US and/or EU; or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
FUNDING
This study was sponsored by Pfizer.
CONFLICT OF INTEREST
P. Emery, AbbVie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, Samsung, Sandoz and UCB (research grants); AbbVie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, Samsung, Sandoz and UCB (consulting fees); F. Breedveld, no disclosures to report; E. Campos, employee of Pfizer and holds stock in Pfizer; A. Szumski, employee of Syneos Health and was contracted by Pfizer to provide statistical support for the development of this manuscript; T. Hirose, employee of Pfizer and holds stock in Pfizer.
ACKNOWLEDGEMENTS
The authors acknowledge the medical writing support provided by David Sunter, PhD, CMPP, of Engage Scientific Solutions and funded by Pfizer.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


