Anti-Arthritics Activity of Cissampelos pareira Leaves and Stephania glabra Rhizome Ethanolic Extract on Adjuvant and Potassium Oxonate Treated Rat
Abstract
Aim:
The study aimed to evaluate the anti-arthritic potential of ethanolic extract of Cissampelos pareira (Menispermaceae) and Stephania glabra (Menispermaceae).
Methods:
Sprague Dawley rats (200 ± 20g) were used as experimental animals. Animal models like Freund’s Adjuvant (FA) induced inflammation, Monosodium Iodo Acetate (MIA) induced osteoarthritis and Potassium Oxonate Induced Uricemia (POU) were used for the study. Total Leukocyte Count (TLC) and Differential Leukocyte Count (DLC) and Tumor Necrosis Factor (TNF-α) were assessed in the blood of rats. The dose of 200 mg/kg of the ethanolic extract of Cissampelos pareira (CPE) and Stephania glabra (SGE) was recorded as the safe dose.
Results:
CPE and SGE significantly decreased (P < 0.001) elevated paw edema on day 7, 14, 21 and 28 in FA-induced arthritis as compared to the control group. Both extracts were found to cause a significant decrease. Also, a significant decrease (P < 0.001) in MIA-induced increase in knee diameter was observed in extracts treated groups. CPE was found to be more effective than SGE. Both extracts were found to be significantly effective (P < 0.001) in potassium Oxonate induced hyperuricemia.
Conclusion:
From the result, it is clear that the ethanolic extracts of CPE and SGE possess beneficial effects against rheumatic disease, osteoarthritis as well as against hyperuricemia. The result may provide an effective treatment against such arthritic problems. Again it may be assumed from the study that the drugs Cissampelos pareira and Stephania glabra can be used to formulate novel drug formulations against the above said ailments.
1. INTRODUCTION
Stephania glabra (Menispermaceae) is a climber vein having large tubers which is up to 30 kg. It grows in the Himalayan region up to an altitude [1]. Cissampelos pareira (Menispermaceae) grows in tropical and subtropical regions of India and is a very famous herbal plant in Indian ‘Ayurvedic’ system. Presently, these plants have been studied for their antiarthritic activities like in treating inflammation leading to rheumatoid arthritis, gout and osteoarthritis.
Inflammation is a part of the host defense mechanisms which leads to the release of several chemical mediators as a result of numerous tissue reactions. Repeated inflammation leads to the formation an of autoantibody which leads to autoimmune diseases. Rheumatoid arthritis is one of such autoimmune diseases.
RA is a long-term disease with dominant extra-articular features and with limited treatment options [2]. Different types of cytokines are known to involve in the pathogenesis of rheumatoid arthritis [3]. Pro-inflammatory cytokines like interferon, interleukin and TNF- α that function in the immune system, are the main members [4]. If we would consider only the synthetic drugs for the treatment of RA, then few medicines like Aspirin, Mephenamic etc. are useful. The serum urate concentration of adult women, which is lower than in men of a similar age is related to higher renal clearance of urate in women, and also estrogen promotes excretion of uric acid during reproductive period [5]. Uric acid is the final product of protein metabolism which, when not excreted from the body through urine, enters in soft tissues of the body in the form of urate crystals. This condition causes pain and when urate crystals get deposited in the joints, they cause difficulty in walking with pain.
Osteoarthritis is wear-and-tear type of degeneration of bones which is associated with pain [6].
In one study, the prevalence rate of arthritis has been shown to increase from 47.8 million cases in 2005 to 67 million cases by 2030 (25% of the adult population), out of which, 25 million cases (9.3% of adult population) have been projected to report arthritis – attributed activity limitation [7].
Several synthetic medicines are being used for treatment and for symptomatic relief of arthritis with a lot of side effects. So, for the safety of patients, the effective medicines from natural sources should be evaluated for the treatment of this disease. There exist some of the plants for gouty arthritis like Allium sativum, Solidagovirgo aurea, Salix fragilis, Tinospora and cardiofolia; Linum usitatissimum, Organum vulgaris, Capsicum annum, and salix alba for osteoarthritis, whereas, Zingiber officinale, Glycyrrhiza glabra, and Curcuma longa for rheumatoid arthritis [8].
To add few more in the above list, the present study is an attempt to evaluate the medicinal potential of CPE and SGE for the treatment of arthritis in experimental animals.
2. MATERIALS AND METHODS
2.1. Plant Material
The leaves of Cissampelos pareira and tubers of Stephania glabra were collected from wild sources during the end of rainy season. Both plants were authenticated in the department of forestry at Dr. YS Parmar University Solan, India. The samples of plants were linked to UHF-Herbarium with Field book number 12544 and 12546 for Cissampelos pareira, and Stephania glabra, respectively. The leaves were dried in shade. The tuber of Stephania glabra was sliced and dried. After drying, both the plants were powdered.
2.1.1. Extraction of Plant Materials
The collected, cleaned and powdered leaves of Cissampelos pareira and dried powdered rhizome of Stephania glabra were taken for extraction. Powdered material of each drug was defatted with petroleum ether first and then marc was evenly packed in the Soxhlet apparatus. Marc was extracted with 95% ethanol. After purification of the solvent, extraction was carried out by hot continuous percolation for about 20 hrs. After extraction, extracts were filtered while hot through a Whatman filter® paper to remove any impurities if present. These extracts were concentrated by vacuum distillation to reduce the volume to 1/10. The concentrated extracts were transferred to 100 ml beaker and the remaining solvent was evaporated on the water bath. Extracts so obtained were then collected and placed in desiccators to remove the excessive moisture. The dried extracts were packed in an airtight container for further studies. The extract of Cissampelos pareira leaves and Stephania glabra rhizome were designated as CPE and SGE respectively.
2.1.2. Preliminary Phytochemical Screening-Preliminary
Phytochemical screening was performed to check the phytoconstituents in the ethanolic extract [9].
2.2. Animals
Sprague Dawley rats (200±20 gm) of either sex were kept under standard environmental conditions (22 ± 2°C under 12 h light and 12 h dark cycles) in separate polypropylene cages in the group of four.
Animals were randomly selected from the animal house of Pinnacle Biomedical Research Institute (PBRI) Bhopal, India. All animals were given standard pellet feeds (golden feed, New Delhi) and drinking water adlibitum throughout the experimental work. The animals were acclimated to laboratory conditions prior to the initiation of the experimental work. Animals were divided into different groups with six animals in each group. The protocol was approved with prior permission of the Institutional Animal Ethics Committee (IAEC) of PBRI (Reg No. - 1283/c/09/CPCSEA).
2.2.1. Acute Oral Toxicity (OECD 423)
CPE and SGE were dissolved in distilled water and administered orally at 5 mg/kg, 50 mg/kg, 300 mg/kg and 2000 mg /kg to different animals as per the guidelines. Mortality was observed as guidelines. None of the animals were dead at 2000 mg/kg, thus it was 1/10th; 200 mg/kg was selected as the dose for present in vivo investigation.
2.2.2. Animal Grouping, Extract Dilution and Application
The rats of either sex were divided into five groups for treating rheumatoid arthritis and hyperuricemia and into four groups for treating osteoarthritis of 6 rats in each.
The ethanolic extracts from each plant were dissolved in normal saline so as to get an anoral dose of 200 mg/kg body weight for groupsIV and V during anti-rheumatoid study. All groups, except Group I, were injected with 0.01 ml Freund’s adjuvant intraperitoneally. Group I animals received normal saline (1 ml/kg) only. Group II animals were kept untreated and served as arthritic control. Group III animals received Indomethacin (10 mg/kg p.o.) and served as a reference standard group. Group IV and V were treated with an oral dose (200 mg/ kg) of CPE and SGE, respectively.
Four groups were opted to check the activity for osteoarthritis. After injecting monosodium iodoacetate (2 mg in a total volume of 50µ intraarticularly) to all the four groups, group I was kept untreated. Group II rats were administered Indomethacin (10 mg/kg given twice a day) orally and designated as the Standard group. Group III rats were provided with CPE (200mg/kg) orally and group IV rats were provided with SGE (200mg/kg) orally.
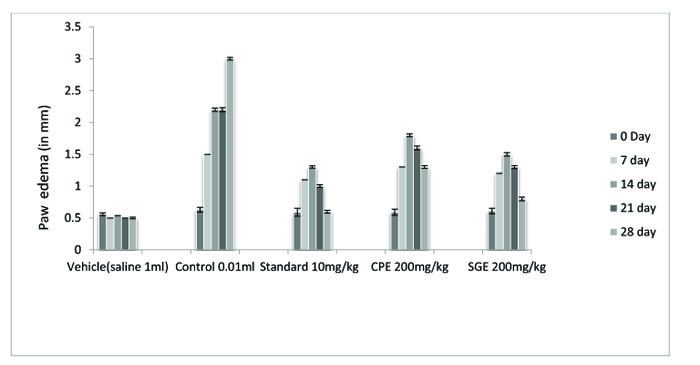
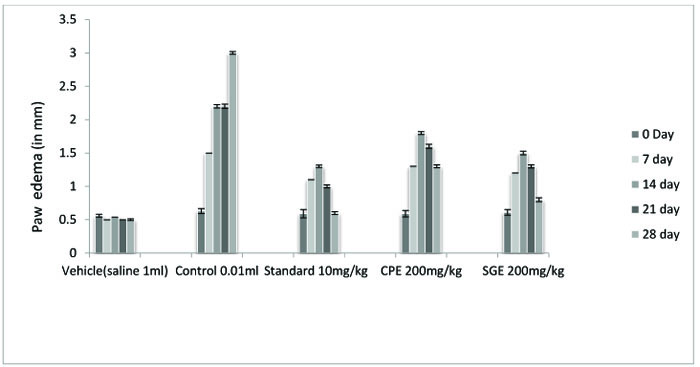
| TREATMENT | DOSE | 7 DAY | 14 DAY | 21 DAY | 28 DAY |
|---|---|---|---|---|---|
| Vehicle | 1 ml | 0.5±0.0190* | 0.54±0.020 | 0.5±0.031 | 0.5±0.026 |
| Control (FA) | 0.01 ml | 1.5±0.0235 | 2.2±0.051 | 2.8±0.062 | 3.0±0.037 |
| Standard (IND) | 10 mg/kg | 1.1±0.0250 | 1.3±0.035* | 1.0±0.042* | 0.6±0.044* |
| CPE | 200 mg/kg | 1.3±0.0180*# | 1.8±0.043* | 1.6±0.058* | 1.3±0.042* |
| SGE | 200 mg/kg | 1.2±0.0150# | 1.5±0.053* | 1.3±0.047* | 0.8±0.055* |
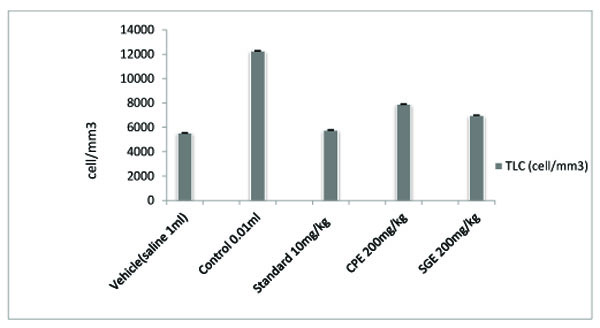
| TREATMENT | DOSE | TLC (cell/mm3) |
|---|---|---|
| Vehicle | 1 ml | 5542.5±43.054* |
| Control (FA) | 0.01 ml | 12268.5±40.203 |
| Standard (IND) | 10 mg/kg | 5777.5±37.572* |
| CPE | 200 mg/kg | 7901.2±39.491* |
| SGE | 200 mg/kg | 6989.7±22.896* |
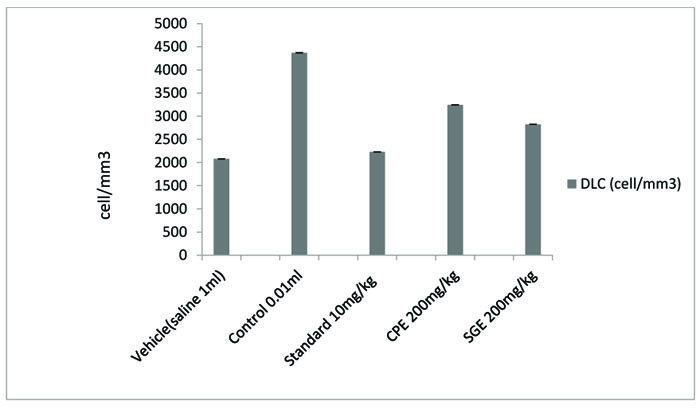
| TREATMENT | DOSE | DLC (cell/mm3) |
|---|---|---|
| Vehicle | 1 ml | 2077.7±41.371* |
| Control (FA) | 0.01 ml | 4369.7±38.003 |
| Standard (IND) | 10 mg/kg | 2228.2±41.242* |
| CPE | 200 mg/kg | 3244.7±34.374* |
| SGE | 200 mg/kg | 2824.2±37.151* |
Five groups were studied to check antihyperuricemic activity. To the rats of group II, III, IV and group V, potassium oxonate was injected intraperitoneally. Saline 1ml/kg was given to Group I rats. Group II rats were remained untreated. Group III rats were given probenecid (45mg/kg p.o.) and tagged as the standard group. To group IV rats, 200mg/kg (orally) CPE and to group V rats, 200mg/kg SGE (orally) were given.
| TREATMENT | DOSE | CONCENTRATION (pg/ml) |
|---|---|---|
| Vehicle | 1 ml | 3.1±0.379 |
| Control (F A) | 0.01 ml | 12.4±0.503 |
| Standard (IND) | 10 mg/kg | 4.6±0.379* |
| CPE | 200 mg/kg | 8.5±0.266* |
| SGE | 200 mg/kg | 6.9±0.167*# |
2.2.3. Arthritis Induction
Freund’s adjuvant induced arthritis- All Rats were anesthetized by intraperitoneal injection of 5% chloral hydrate at a dose of 0.25 mg /kg body weight. Then, to induce arthritis, 0.01 ml Freund’s adjuvant was injected intraperitoneally into the plantar region of the right hind paw of each rat [10, 11].
Osteoarthritis induced by Monosodium Iodo Acetate (MIA)- Monosodium iodoacetate was injected intra-articularly in a total volume of 50 μl (2 mg) through the patellar ligament of the right knee for the induction of experimental osteoarthritis, using a 26-gauge needle, in anesthetized rats. The left knee joint (control) was injected with saline. Oral treatment with extract (200 mg/kg) was started on day 7 after MIA injection and was continued until the termination of the study. Another group of rats was administered with indomethacin (10 mg/kg) orally. The control group was treated with vehicle alone [12].
Hyperuricemia was induced by injecting rats intraperitoneally with potassium oxonate (200 mg/kg) and natrium urate (15 mg/kg) orally 1 h before the final drug administration to increase the serum urate level.
2.2.4. Measurement of Paw Volume and Knees Diameter
Measurements of the paw volumes were started on day 0, 7, 14, 21and 28 using a digital caliper. Rats were otherwise observed daily.
For osteoarthritis, the diameter of the knees was measured every other day using electronic digital calipers. The readings were taken on 14, 16, 18, 20 and 22 days after the disease induced.
2.3. Collection of Blood Sample
After 28 days, blood samples were collected by puncturing the retro-orbital plexus into heparinized vials and used for further study.
For the anti-hypouricemic study, whole blood samples were collected from rats by tail vein bleeding on 2, 4, 6 and 8 hours after the induction of arthritis. The blood was then centrifuged at 12000 rpm to obtain the serum. The serum so obtained was stored at -20oC until assayed.
2.4. Determination of Serum Inflammatory Markers
Total Leukocyte Count (TLC) and Neutrophil count (DLC) were examined in blood samples of the treated and untreated group [13]. The serum TNF-α was examined to estimate the concentration of this proinflammatory cytokine by using a standard ELISA kit (‘GENXBIO-make’) for TNF-α.
Serum uric acid level was determined by Biochemical autoanalyzer (STAR 21, Span Diagnostic Ltd).
2.5. Statistical Analysis
Single line ANOVA was applied to test the significance of differences between the results of extract-treated and control and standard groups. The results were expressed as Mean± SEM value. The difference was considered significant at the conventional level of significance (P < 0.001)
3. RESULT AND DISCUSSION
Alkaloids, tannins, Flavonoids, carbohydrates and phenols were reported to be present in the ethanolic extract of CPE whereas, amino acids, alkaloids, flavonoids, carbohydrates, phenols and saponin were found to be present in the ethanolic extract of SGE.
The ethanolic extract of leaves of CPE and tubers of SGE was found to be safe at a dose of 200 mg/ kg. Anti-arthritic activity was evaluated according to the ability of test and standard extract to inhibit Freund's Adjuvant-induced paw swelling, the concentration of total leukocytes count, differential leukocytes count and TNF-α. After the induction of arthritis, the increase in swelling was observed. The diameter of paw volume was measured of the rats of each group on 0, 7th, 14th, 21th and 28th day after the induction of arthritis. The result so obtained was compared with that of the results of the arthritic control group and standard group (Fig. 1, Table 1).
All the groups showed a significant (P < 0.001) decrease in the paw volume after treatment. Comparison between SGE (1.2 ± 0.0150) and CPE (1.3 ± 0.0180) showed the insignificant result on day 7, similarly on the same day when SGE (1.2±0.0150) results were compared with the standard (1.1 ± 0.0250), significant (P < 0.05) results were obtained. Both CPE and SGE were reported to be significant with the vehicle group (group I). Total Leukocyte Count (TLC) shown in Fig. (2), Table 2 and Differential leukocyte count (DLC) in Fig. (3), Table 3 were examined in the serum of all groups. Blood samples were examined with respect to total leukocyte and neutrophil count in cell/mm3. The concentration for each group so obtained were compared with the control and standard group. All treated groups were found to show a significant decrease in the TLC and DLC each day except day 0.
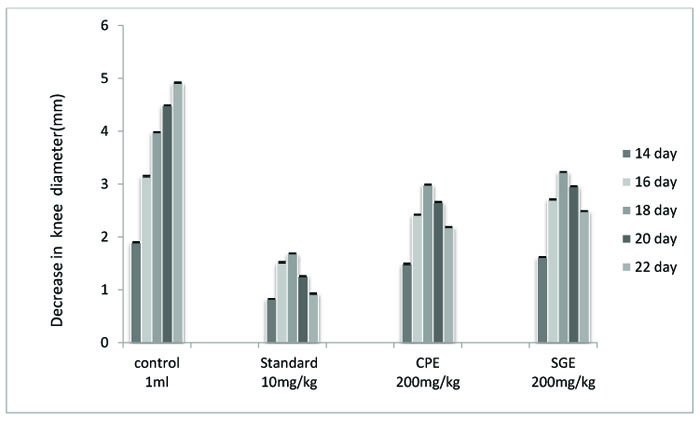
| TREATMENT | DOSE | 14 DAY | 16 DAY | 18 DAY | 20 DAY | 22 DAY |
|---|---|---|---|---|---|---|
| Vehicle | 1 ml | 1.90±0.0150 | 3.15±0.0250 | 3.98±0.0141 | 4.49±0.0095 | 4.92±0.0221 |
| Standard (IND) | 10 mg/kg | 0.83±0.0095*,# | 1.52±0.0263*,# | 1.69±0.0095*,# | 1.26±0.0125*,# | 0.93±0.0208*,# |
| CPE | 200 mg/kg | 1.49±0.02348*,# | 2.42±0.0129*,# | 2.99±0.0170*,# | 2.66±0.0182*,# | 2.19±0.0129*,# |
| SGE | 200 mg/kg | 1.62±0.0170* | 2.71±0.0170* | 3.23±0.0095* | 2.96±0.0129* | 2.49±0.0129* |
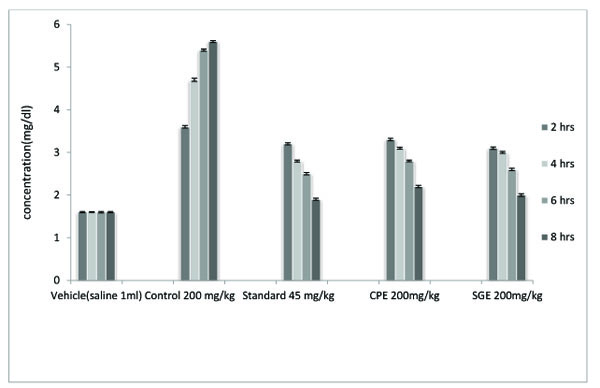
| TREATMENT | DOSE | 2 Hrs | 4 Hrs | 6Hrs | 8 Hrs |
|---|---|---|---|---|---|
| Vehicle | 1 ml | 1.6±0.031*,&,# | 1.6±0.021*,&,# | 1.6±0.033*,# | 1.6±0.030*,# |
| Control (Pot. oxonate) | 200 mg/kg | 3.6±0.061 | 4.7±0.071 | 5.4±0.049 | 5.6±0.045 |
| Standard (Probenecid) | 45 mg/kg | 3.2±0.047*,& | 2.8±0.043*,& | 2.5±0.0531* | 1.9±0.051* |
| CPE | 200 mg/kg | 3.3±0.056* | 3.1±0.046* | 2.8±0.038* | 2.2±0.053* |
| SGE | 200 mg/kg | 3.1±.0.053*,& | 3.0±0.046*,& | 2.6±0.051* | 2.0±0.058* |
The concentration of TNF- α in pg/ml was examined as compared to vehicle control, Freund's adjuvant control, standard and extracts (Fig. 4, and Table 4). All treated groups showed a significant (P < 0.001) decrease in the paw volume. The comparison between CPE and SGE and standard vs vehicle also showed a significant decrease (P < 0.05) in paw volume. to the untreated control group showed a remarkable decrease in the knee diameter but not in comparison with standard indomethacin group. CPE proved to be more effective against monosodium iodoacetate induced osteoarthritis than SGE (Fig. 5, Table 5).It becomes clear from the examination of blood samples that both the extracts CPE and SGE significantly (P < 0.001) decrease the elevated uric acid level. The significant results were obtained in comparison to the control group II but not with Standard group III (Fig. 6 and Table 6).
Both CPE and SGE showed a significant (P <0.001) decrease in the knee diameter. CPE and SGE in the comparison
In the present study, the antiarthritic activity of Cissampelos pareira and Stephania glabra was evaluated for their ethanolic extracts. Similarly, anti-inflammatory activity on Cissampelos pareia has been reported by using different solvents for extraction and different models [14, 15].
Ethanolic extract of Cissampelos pareira has been tested against carrageenan-induced inflammation and it was found to be 59.5% and 64.04% effective for acute inflammation. However, the use of Cissampelos pareira has also been mentioned in Panchakarma Ayurvedic therapy [16]. In folklore medicine system, decoction of Stephania glabra has been used for the treatment of rheumatic body ache and fever [17]. Role and interest of herbal drugs in the treatment of rheumatoid diseases is well established. For example, an antirheumatic in vitro and in vivo study on Eupatorium purpureum has been reported in which Cistifolin was found to be present as the main constituent. Cistifolin inhibits the Mac-ic1 (CD11b / CD18)-dependent monocyte adhesion to fibrinogen [18]. In one more study, extracts from stinging nettle (Urtica dioica), were reported to show an inhibitory action on the proinflammatory transcription factor NF-κB which is one of the causes of rheumatic disease [19].
Here, for detrmining anti-inflammatory activity, all the groups were first injected with 0.01ml Freund’s adjuvant. SGE was observed to show better results than CPE. On day 14, 21 and 28, significant (P < 0.001) results were obtained. On day 28, significant results were obtained at P < 0.05 between CPE and SGE.
The role of herbal drugs for the treatment of osteoarthritis [20-22] has been evaluated by several researchers. Those previous researches on the treatment of osteoarthritis have shifted the attention from conventional drugs towards safer herbal intervention. Here, by taking this fact into consideration, the ethanolic extracts of two of such medicinal plants namely Cissampelos pareira (CPE) and Stephania glabra (SGE) were studied against monosodium iodoacetate induced osteoarthritis and significant (P < 0.001) results were obtained. CPE was found to be more effective in reducing the raised diameter of the knee. Both CPE and SGE decrease the knee diameter (in mm) in comparison with the untreated control group but showed less decrease in the knee diameter than indomethacin standard group.
Significant(P < 0.001) results were obtained for TLC, DLC and TNF- α. SGE treated rats of Group V were found to have less concentration of TLC and DLC (cell/mm3) than CPE treated rats of group IV. Group V showed significant (P < 0.001) results with control and standard group. Similarly, in rats treated with SGE, a higher decrease in elevated concentration of TNF- α (pg/ml) was obtained by using a standard ELISA kit (‘GENXBIO-make’). The decrease in the concentration of TNF- α was higher in Group V.
Uric acid concentration (mg/dL) was assessed in the blood serum sample after 2, 4, 6 and 8 hours of injection of potassium oxonate. A significant (P < 0.001) decrease in the uric acid level was obtained in comparison to the untreated control group. Both CPE and SGE showed less effectiveness than the standard group. In the result of 2 hrs, SGE showed more decrease in uric acid concentration than standard probenecid treated group. SGE was found to decrease a higher amountof uric acid than did CPE . SGE and CPE did not show significant results after 4 hours.
Overall, SGE was found to be more effective than CPE in decreasing Freund's adjuvant-induced paw volume, uric acid concentration and in lowering the concentration of TLC, DLC and tumor necrosis factor alpha (TNF-α). Whereas CPE was found to be more effective in decreasing the experimentally raised knee diameter. The significant action of CPE and SGE on leukocytes count may be due to the presence of flavonoids in these extracts.
Similarly, Crataegus oxyacantha fruit extract has been reported to exert a significant effect on DLC count. Especially, the decrease in neutrophils and an increase in lymphocytes were reported because of the high concentration of flavonoids in the Crataegus oxyacantha extract [23]
CONCLUSION
From the above experiment, it can be concluded that the ethanolic extract of stems of leaves of Cissampelos pareira and tubers of Stephania glabra possesses significant (P < 0.001) anti-inflammatory activity in rheumatoid arthritis, Osteoarthritis against experimentally induced arthritis in rats and against experimentally induced hyperuricemia. This may be due to the presence of active constituents and their influence on the release of cytokines and other chemical mediators at the site of inflammation. Further research to isolate the anti-inflammatory principle and the exact mechanism involved, is needed.
LIST OF ABBREVIATIONS
| CPE | = Cissampelos Pareira Extract |
| SGE | = Stephania Glabra Extract |
| TLC | = Total Leucoyte Count |
| DLC | = Diffrential Leucocyte Count |
| TNF-α | = Tumer Necrosis Factor- Alpha |
| ELISA | = Enzyme Linked Immuno Sorbent Assay |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All animal experiments were performed with prior permission from the Institutional Animal Ethics Committee (IAEC) of Pinnacle Biomedical Research Institute (PBRI) Bhopal (Reg No. 1283/c/09/CPCSEA). Protocol Approval Reference No. PBRI/IAEC/12/PN-226.
HUMAN AND ANIMAL RIGHTS
No humans were used in this research all research procedures were carried out in accordance as laid in the guidelines of 'Guide for the care and use of Laboratory Animals'.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
We are thankful to Pinnacle biomedical research institute (PBRI) Bhopal, India to provide us all lab facilities and Dr. YS Parmar, Solan University (HP), India in authenticating the plants.


