All published articles of this journal are available on ScienceDirect.
Predictors of Remission Maintenance after Etanercept Tapering or Withdrawal in Early Rheumatoid Arthritis: Results from the PRIZE Study
Abstract
Objective:
To explore the influence of early treatment response to etanercept-methotrexate therapy on sustained remission after tapering/withdrawal of etanercept in methotrexate/biologic-naïve patients with early rheumatoid arthritis in the PRIZE study (ClinicalTrials.gov: NCT00913458).
Method:
In the initial 52-week open-label phase, enrolled patients received once-weekly etanercept 50 mg plus methotrexate. Patients who achieved DAS28 ≤3.2 at week 39 and <2.6 at week 52 were randomized to etanercept 25 mg plus methotrexate, methotrexate monotherapy, or placebo once weekly for 39 weeks in the double-blind phase. The relationships between responses in the open-label phase and sustained remission (DAS28 <2.6 at weeks 76 and 91, without glucocorticoid rescue therapy from weeks 52 to 64) in the double-blind phase were analyzed.
Results:
In the open-label phase, 70% of patients achieved DAS28 remission at week 52. In the double-blind phase, 63%, 40%, and 23% of patients had sustained DAS28 remission in the reduced-dose combination-therapy, methotrexate-monotherapy, and placebo groups, respectively. In patients receiving reduced-dose combination therapy, sustained remission was more likely in those who achieved DAS28 remission (p = 0.005) or low disease activity (p=0.044) in a shorter time, and who had a lower DAS28 (p = 0.016) or achieved ACR/EULAR Boolean remission (p < 0.05) at the end of the open-label phase. In patients receiving methotrexate monotherapy, sustained remission was associated with a lower acute-phase response (C-reactive protein, p = 0.007; erythrocyte sedimentation rate, p = 0.016) at the end of the open-label phase.
Conclusion:
Fast response and suppression of inflammation with etanercept-methotrexate therapy may predict successful etanercept tapering/withdrawal in patients with early rheumatoid arthritis.
1. INTRODUCTION
Clinical remission and Low Disease Activity (LDA) are currently well-recognized treatment targets for patients with Rheumatoid Arthritis (RA), regardless of the level of their disease activity, because control of inflammation has been shown to reduce structural joint damage, quality-of-life impairment, and functional disability [1-3]. The effectiveness of combination therapy with an anti-Tumor Necrosis Factor (TNF) agent and the conventional Disease-Modifying Antirheumatic Drug (DMARD) methotrexate in early RA has been demonstrated over decades of clinical research [4-9]. In patients who achieve remission, clinicians may consider biologic dose reduction or complete withdrawal due to concerns regarding adverse event risk and cost [10], but such an approach requires an understanding of the factors that influence remission maintenance after de-escalation of anti-TNF therapy. However, identification of patients who may be most likely to achieve a sustained response with a maintenance regimen remains a challenge, as little definitive evidence on predictive markers is available.
During the open-label first phase of the PRIZE (Productivity and Remission in a randomIZed controlled trial of etanercept vs standard of care in Early rheumatoid arthritis) study, patients with early, active, moderate-to-severe RA showed significant and rapid improvement in disease activity and function with combined 50 mg etanercept once weekly (QW) with methotrexate therapy [11]. In this early RA population, induction therapy with the combination regimen was associated with remission or LDA in most of the treated patients. In the double-blind second phase, patients with qualifying responses at weeks 39 and 52 of the open-label phase were randomized to receive either combination 25 mg etanercept plus methotrexate, methotrexate monotherapy, or placebo QW for 39 weeks. Better disease control was maintained by continuing the combination regimen, albeit with a reduced biologic dose, than by switching to methotrexate monotherapy or placebo. The design and patient populations of the PRIZE study provided the opportunity to examine factors that may have influenced the maintenance of remission in the double-blind phase after etanercept was tapered or withdrawn among patients who initially responded to induction therapy with the full-dose regimen.
2. PATIENTS AND METHODS
The population and methodology of the PRIZE study (ClinicalTrials.gov identifier: NCT00913458) have been described in detail in a previous publication [11]. In brief, eligible patients had moderate-to-severe RA, symptom onset within 12 months of enrollment, and no prior exposure to methotrexate or biologic agents. In the initial open-label phase (Supplemental Fig. S1), patients received subcutaneous (SC) etanercept 50 mg plus oral methotrexate 10 mg (up to a maximum dose of 25 mg) QW for 52 weeks. Patients who met the criteria for response, defined as Disease Activity Score for 28-joint counts (DAS28) LDA at week 39 and DAS28 <2.6 (i.e., remission) at week 52 were randomized to receive SC etanercept 25 mg plus oral methotrexate (i.e., combination therapy), oral methotrexate plus SC placebo (i.e., methotrexate monotherapy), or SC and oral placebo (i.e., placebo) during the subsequent 39-week double-blind phase. At weeks 12, 24, and 39 of the double-blind phase, patients with a DAS28 >3.2 were withdrawn from the study. Randomized treatment was discontinued in patients who achieved DAS28 LDA at week 39 of the latter phase, and these patients were observed through the final 26-week withdrawal phase of the study. Radiographs of the hands, wrists, and feet were taken at baseline of the open-label phase and the start and end of the double-blind phase; the images were assessed by a central blinded reader and scored based on the van der Heijde modification of total Sharp score (modified Total Sharp Score [mTSS]).
In prespecified exploratory and post hoc analyses, patients’ demographic and disease characteristics at baseline and treatment responses in the open-label phase were evaluated as predictors of sustained remission in the double-blind phase. Sustained remission was defined as achievement of DAS28 <2.6 at weeks 76 and 91, without the need for glucocorticoid rescue therapy from weeks 52 to 64. Baseline demographic and disease characteristics included in these analyses as predictive variables were age and sex; disease duration; prior use of corticosteroids or nonsteroidal anti-inflammatory drugs; anti-Cyclic Citrullinated Peptide antibody (aCCP) and Rheumatoid Factor (RF) status; C-Reactive Protein (CRP) level and erythrocyte sedimentation rate (ESR); tender and swollen joint counts; DAS28; scores on the Health Assessment Questionnaire (HAQ), Physician and Subject Global Assessments (PGA and SGA), pain Visual Analog Scale (VAS), and Medical Outcomes Short Form-36 Health Survey; and mTSS. Treatment responses at week 52 of the open-label phase that were included in the analyses as predictive variables were CRP levels and ESR; DAS28 remission and LDA; remission according to American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) Boolean criteria; DAS28; scores on HAQ, PGA, SGA, pain VAS, and Functional Assessment of Chronic Illness-fatigue; and the mTSS.
These analyses were conducted in the double-blind modified Intent-To-Treat (mITT) population, defined as all patients who had received ≥1 dose of treatment in the double-blind phase and had ≥1 DAS28 assessment after randomization, using nonresponder imputation (i.e., all patients who dropped out of the study were identified as nonresponders). Relationships among baseline characteristics, early treatment outcomes, and sustained remission were analyzed using Odds Ratios (ORs) from univariate logistic regression models, with treatment as factor; Mantel–Haenszel chi-square correlation trend test (univariate analyses of quartiles of response); and stepwise logistic regression.
3. RESULTS
3.1. Patient Disposition
Of the 306 patients enrolled in the PRIZE study, 222 completed the 52-week open-label phase, and 198 achieved a treatment response with full-dose combination therapy (Supplemental Fig. S1) [11]. Of the responders, 193 entered the double-blind phase and were randomized to treatment with either reduced-dose combination etanercept-methotrexate therapy (n = 63), methotrexate monotherapy (n = 65), or placebo (n = 65), and 132 achieved a response after 39 weeks of double-blind treatment.
3.2. Response in the Open-Label and Double-Blind Phases
In the open-label phase, in which all patients received etanercept 50 mg plus methotrexate, 70%, 61%, 57%, and 51% of patients achieved remission according to the DAS28, Simplified Disease Activity Index (SDAI), Clinical Disease Activity Index (CDAI), and ACR/EULAR Boolean criteria, respectively, at week 52 (Fig. 1A). A total of 78%, 81%, and 81% of patients attained LDA based on DAS28, SDAI, and CDAI, respectively, and 67% attained normal HAQ-DI. Among patients who met response criteria and were randomized to treatment in the double-blind phase, 63%, 40%, and 23% in the reduced-dose combination therapy, methotrexate monotherapy, and placebo groups, respectively, achieved sustained DAS28 remission by the end of the double-blind phase (Fig. 1B; combination therapy vs. methotrexate, p<0.01 and vs. placebo, p<0.0001).

3.3. Predictors of Sustained Response in the Double-blind Phase
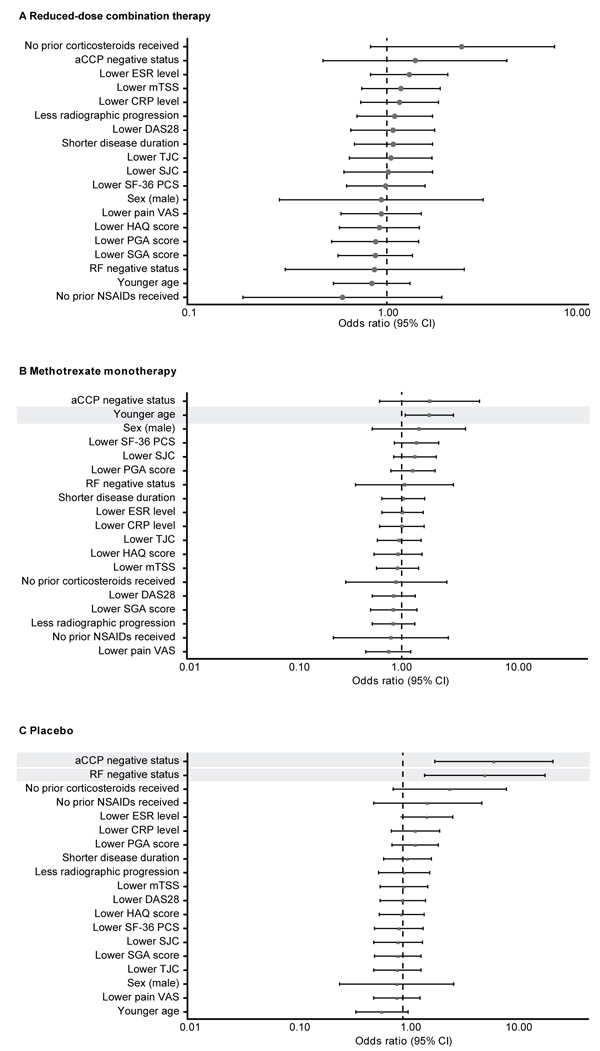
None of the baseline demographic or disease characteristic predictors analyzed was found to predict sustained remission with the reduced-dose combination regimen (etanercept 25 mg plus methotrexate) in the double-blind phase (Fig. 2A). Sustained remission with methotrexate monotherapy was significantly more likely in younger patients (p = 0.028; Fig. 2B), and with placebo, in those who were negative for aCCP (p = 0.002) or RF (p = 0.007) (Fig. 2C).
Sustained remission with the reduced-dose combination therapy was significantly more likely in patients who had achieved DAS28 remission (p=0.005) or LDA (p=0.044) in a shorter time with induction therapy and who had a lower DAS28 (p=0.016; Fig. 3A) at the end of the open-label phase. With methotrexate monotherapy, sustained remission was associated with lower CRP (p=0.007) or ESR (p=0.016) levels at week 52 (Fig. 3B). No early response predictors of sustained remission were found with placebo (Fig. 3C).
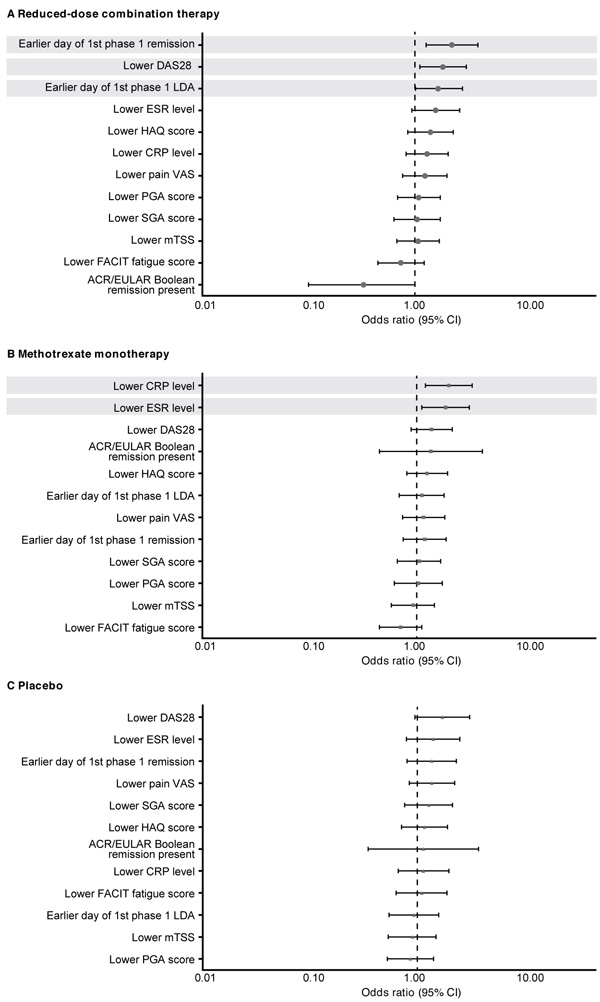
In post hoc univariate analyses, a significantly higher proportion of patients who achieved sustained remission with reduced-dose combination therapy had achieved first DAS28 remission (p<0.01) or LDA (p<0.05) in a shorter time with induction therapy and had a lower DAS28 at week 52 (p<0.05; Table 1). Sustained remission with the reduced-dose regimen was also significantly more likely among patients who achieved ACR/EULAR Boolean remission at week 52 with induction therapy compared with those who did not (71% vs. 44%; p<0.05). Patients who achieved sustained remission with methotrexate monotherapy were more likely to have been younger (p<0.05) and to have had lower CRP levels at baseline (p <0.01; Table 1). In the methotrexate monotherapy group, sustained remission was achieved by 49% of patients who had DAS28 LDA from weeks 13 to 52 of the open-label phase compared with 23% of those who did not (p <0.05). Sustained remission with placebo was reported in higher proportions of patients who had been seronegative at baseline for aCCP (seronegative vs. seropositive: 48% vs. 11%; p≤0.01) and for RF (41% vs. 11%; p <0.01) antibodies.
| Predictor | Quartiles of Response, n/N (%) | p-value* | |||
|---|---|---|---|---|---|
| Reduced-Dose Combination Therapy | |||||
| First DAS28 remission, day | 13–57 | 58–179 | 180–273 | ≥274 | |
| n/N (%) | 12/15 (80) | 16/20 (80) | 8/16 (50) | 4/12 (33) | <0.01 |
| First DAS28 LDA, day | 1–29 | 30–57 | 58–183 | ≥184 | |
| n/N (%) | 12/14 (86) | 12/20 (60) | 10/15 (67) | 6/14 (43) | <0.05 |
| Mean DAS28, week 52 | ≤1.55 | >1.55−1.91 | >1.91−2.16 | >2.16 | |
| n/N (%) | 14/16 (88) | 11/16 (69) | 7/15 (47) | 8/16 (50) | <0.05 |
| Methotrexate Monotherapy | |||||
| Age, BL, years | 19–39 | 40–48 | 49–60 | ≥61 | |
| n/N (%) | 11/15 (73) | 8/22 (36) | 2/15 (13) | 5/13 (38) | <0.05 |
| CRP level, week 52, mg/L | 0–0.50 | >0.50–1.09 | >1.09–2.91 | >2.91 | |
| n/N (%) | 9/16 (56) | 8/14 (57) | 6/18 (33) | 2/16 (13) | <0.01 |
BL: baseline; CRP: C-Reactive Protein; DAS28: Disease Activity Score for 28-joint counts; LDA: Low Disease Activity.
When the relationship between baseline demographic and disease characteristics and sustained remission in the double-blind phase was analyzed using stepwise logistic regression, sustained remission in the placebo group was more likely among patients who had been seronegative versus seropositive for aCCP antibody at baseline (OR, 7.799; 95% confidence interval [CI] 2.171–28.015). In analyses of early treatment response and sustained remission, patients who achieved DAS28 remission in a shorter time with induction therapy were more likely to maintain remission in the reduced-dose combination therapy group (OR, 1.008; 95% CI 1.003-1.014); patients who had lower ESR levels after induction therapy were more likely to have sustained remission with methotrexate monotherapy (OR, 1.171; 95% CI 1.033-1.328).
4. DISCUSSION
Recent EULAR recommendations for the treatment of RA [3] suggest that induction therapy with a biologic plus conventional DMARD followed by tapering or discontinuation of the biologic and continuation of the conventional DMARD may be a valuable approach, which is supported by multiple reports in the literature [11-16]. However, the EULAR Committee advised that biologics should be tapered primarily when used in combination with conventional DMARDs to achieve sustained remission, as their discontinuation frequently results in a recurrence of disease activity [17, 18]. Although a few predictors of successful biologic tapering in initial responders have been identified [10], such characteristics have not yet been adequately studied.
In the open-label induction phase of the PRIZE study, patients who had not previously been treated with methotrexate or biologic therapy showed significant and rapid improvement in clinical and functional outcomes with full-dose etanercept-methotrexate combination induction therapy. Among patients who responded to induction therapy, remission was more effectively maintained with a reduced-dose combination therapy regimen than with a biologic-free regimen in the double-blind phase. In the latter maintenance phase, early onset of response to induction therapy with the full-dose regimen was an important predictor of subsequently sustained remission after tapering of etanercept. The key factors predicting patients’ achievement of sustained remission with reduced-dose combination maintenance therapy were early first remission, early first LDA, low DAS28, and ACR/EULAR Boolean remission with induction therapy. Predictors of biologic-free sustained remission after etanercept withdrawal, i.e., in the methotrexate monotherapy and placebo groups, included younger age and aCCP– / RF– status at baseline, and lower acute-phase response and early persistent response after open-label treatment with the full-dose combination regimen.
Our findings that short-term treatment response is predictive of future outcomes are consistent with those of multiple other studies [19-26], although the aforementioned analyses of predictors of biologic-free remission are unique. In a post hoc analysis of the PREMIER study, patients with early, aggressive RA who had an early, more robust response to methotrexate monotherapy demonstrated more favorable clinical, functional, and radiographic long-term outcomes than those with a delayed, less robust response [26]. Patients with an early or higher magnitude response to methotrexate/anti-TNF combination therapy showed less difference in long-term outcomes, although some upward trends were observed. In the TEAR trial, conducted in methotrexate-refractory patients with moderate-to-severe early RA, clinical response achieved within 3 months of initiating or escalating conventional DMARD/biologic therapy was shown to be a reliable predictor of LDA at 1 year [24].
The results of the PRIZE study, which support an intensive treatment strategy targeting achievement of DAS28 or ACR/EULAR Boolean remission in patients with early RA prior to biologic tapering or withdrawal, also build on those of other randomized, controlled, double-blind clinical trials that have demonstrated the benefits of early intervention [6, 27, 28]. In the Combination of Methotrexate and Etanercept in Early Rheumatoid Arthritis (COMET) trial, a significantly greater proportion of patients in the etanercept-methotrexate combination therapy group achieved DAS28 remission after 52 weeks of treatment compared with patients in the methotrexate monotherapy group (50% vs. 28%; p < 0.0001) [28]. Importantly, remission rates observed in the COMET population (mean disease duration, 9.0 months) were ~10% higher than those reported in the TEMPO study with combination and methotrexate therapies in a population with more established RA (mean disease duration, 6.8 years) [29]. In addition, post hoc analysis of COMET trial data revealed that treatment with etanercept plus methotrexate provided qualitatively better clinical outcomes in very early RA (disease duration ≤ 4 months) than in early RA (> 4 months to 2 years): DAS28 remission was achieved in 70% versus 48% (p <0.05) of patients in the very early versus early RA groups, respectively [30]. These high remission rates were not observed with methotrexate monotherapy (35% vs. 32%, respectively; p>0.70).
Limitations of the PRIZE study have been previously presented [11]. This study was designed to examine whether the efficacy of full-dose etanercept-methotrexate induction therapy would be maintained after the reduction or withdrawal of the biologic in patients with early, moderate-to-severe RA who previously responded to induction therapy. Because no patients received full-dose combination therapy in the double-blind phase, we could not directly compare the efficacy of dose tapering or discontinuation with that of continuing full-dose therapy in this population. Many of the analyses in the current report were based on post hoc models, with no multiple comparison control. Moreover, our findings may not be reproducible and should not be considered generalizable or predictive. The predictors reported in the current paper were derived from patients who had not received previous methotrexate or biologic therapy.
CONCLUSION
In patients with RA, identification of baseline characteristics and early clinical outcomes that predict future response to maintenance therapy with conventional DMARD and/or biologic therapy may improve clinicians’ decision-making when managing this chronic, potentially disabling disease. A patient’s demographic and disease features and early response may help inform the clinician’s choice to either stay the course with the originally selected therapeutic agent or regimen; modify the original approach via addition, subtraction, or dose adjustment; or switch to an entirely different agent or regimen, in the pursuit of remission or an LDA state. Our results and those reported previously are clinically relevant, as they may allow more timely therapeutic adjustments and better long-term outcomes. They suggest that an early aggressive treat-to-target approach may be best if subsequent de-escalation of biologic therapy is desired. In the practice setting, clinicians may consider patients who achieve a rapid clinical response, ACR/EULAR Boolean remission, and/or more complete suppression of inflammatory markers with full-dose etanercept-methotrexate therapy as the most appropriate candidates for a trial of biologic dose reduction or withdrawal. However, additional research is needed to detect not only single predictors of treatment response, but also sets of multiple predictors that will improve management of early and established RA of patients in clinical practice.
AUTHOR CONTRIBUTIONS
All authors contributed to the conception and design of the predictor analyses from the PRIZE study and were involved in interpreting the data and reviewing, revising, and developing the manuscript. All authors also approved the final version of the article that was submitted for publication. All authors had full access to all of the data and take responsibility for its integrity and accuracy. The article’s publication was not contingent on approval by Pfizer.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Review Board and ethics committee at all participating centers approved the study protocol.
HUMAN AND ANIMAL RIGHTS
The study was conducted according to the ethical principles of the Declaration of Helsinki and the Good Clinical Practice guidelines of the International Conference on Harmonisation.
CONSENT FOR PUBLICATION
All authors provided consent for the publication of the submitted manuscript.
CONFLICT OF INTEREST
This study was sponsored by Pfizer. Prof. Emery reports receiving grant/research support and consultancy fees from AbbVie, BMS, Lilly, MSD, Novartis, Pfizer, Roche, Samsung, Sandoz, and UCB. Mr. Pedersen, Dr. Bukowski, and Dr. Marshall are Pfizer employees and owners of Pfizer stock.
ACKNOWLEDGEMENTS
The authors thank all patients, investigators, and medical staff members who were involved in the PRIZE study. Editorial/medical writing support was provided by John Bilbruck, Samantha Forster, PhD, Lorna Forse, PhD, and Donna McGuire of Engage Scientific Solutions and was funded by Pfizer.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.


