All published articles of this journal are available on ScienceDirect.
Serum Level of Endothelial Cell-Specific Molecule -1 (ESM -1) as a New Potential Biomarker for Rheumatoid Arthritis Disease Activity
Abstract
Background:
Rheumatoid arthritis is a chronic inflammatory autoimmune disease characterized by destruction of the joint cartilage and bone. Endothelial dysfunction (ED) in RA may be related to disease activity. Our objective is to explore serum levels of endothelial cell-specific molecule-1 (ESM-1) as a biomarker for RA disease activity.
Methods:
A cross-sectional study was carried out and included 83 adult patients with RA, in addition to 20 healthy subjects (age and sex-matched) as a control group. Based on Disease Activity Score in 28 joints (DAS-28), the patient's group was subdivided into four subgroups(remission, mild, moderate and severe disease activity state). The demographic & clinical data, BMI, DAS-28 and Serological assessment [Erythrocyte Sedimentation Rate (ESR), CRP, Rheumatoid Factor (RF) and Anti-Citrullinated Peptide Antibody (ACPA)] were recorded. ESM-1was assayed for all participants.
Results:
Serum levels of ESM1 were significantly higher in the patient group than the control group (P < 0.0001). ESM-1 serum levels were significantly higher in patients with severe disease activity subgroup compared with patients with remission and mild disease activity subgroups (P < 0.0001). ESM-1 was positively and significantly correlated with DAS-28 score, The Health Assessment Questionnaire Disability Index (HAQ-DI) and modified Larsen score (P = 0.002, 0.0001 & 0.0001 respectively).
Conclusion:
ESM-1 could be a biomarker for RA disease activity.
1. INTRODUCTION
Rheumatoid Arthritis (RA) is a chronic auto-inflammatory disease [1]. Evaluation of Disease progression in RA is based on certain parameters including clinical disease activity indices (e.g. DAS -28), radiological, and specific serological biomarkers. The laboratory markers, specifically Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP), are not specific and are influenced by several other variables [2]. Endothelial cells play a vital part in the initiation of the inflammatory process [3]. Endothelial Dysfunction (ED) in RA may be linked to the Functional defect of endothelial progenitor cells, which are included in vasculogenesis and vascular repair [4]. However, the exact pathophysiological mechanisms of ED in RA are still ill-defined [5]. Definitely, numerous markers of endothelial cell abnormalities are linked with the inflammatory progression seen in RA [6]. Endothelial cell-specific molecule-1 (ESM-1) is a significant immunoregulator glycoprotein secreted by endothelial linings of the lungs and kidneys [7, 8] and can be detected in healthy persons [9]. ESM-1 is used as a biomarker for endothelial cell dysfunction [10, 11] and has been identified as a key player in inflammatory conditions [12-14]. Levels of ESM-1 were elevated in synovial tissue of RA patients causing angiogenesis and share in pannus formation [15]. Whether serum levels of ESM-1 are associated with endothelial dysfunction or disease activity in patients with RA, at this point, we were targeting to explore serum levels of ESM-1 as a new potential biomarker for RA disease activity.
2. METHODS
Our cross-sectional study was designed to include 83 adult patients with RA from OPC of rheumatology and immunology clinic, Mansoura University hospitals from June to Dec. 2016 (RA patients were identified who satisfied the 2010 ACR diagnostic criteria) [16]. Exclusion criteria included the history of cancer, any history of cardiovascular disorders, hematological abnormality, any acute or other infection, a granulomatous chronic disease, Inflammatory Bowel Diseases (IBD), a metabolic disease, other autoimmune disorders, DM and anti-TNF usage (anti-TNF therapy). The study protocol had an approval from the Ethics Committee of the School of Medicine, Mansoura University (R1/16.10.06). The study cohort was divided into four subgroups according to DAS-28 score: 15 patients in remission (ACR/EULAR 2011 remission criteria [17] (DAS-28 < 2.6), 15 patients with low disease activity (DAS28: 2.6 --3.2), 38 patients with moderate disease activity (DAS-28: 3.2--5.1) and 15 patients with high disease activity (DAS-28 > 5.1). In addition to 20 age and sex matched healthy subjects, were included as a control group. All demographic & clinical data, BMI, Disease Activity Score in 28 joints (DAS-28) and Serological assessment comprised Erythrocyte Sedimentation Rate (ESR), CRP, Rheumatoid Factor (RF) and Anti-Citrullinated Peptide Antibody (ACPA) were recorded. The Health Assessment Questionnaire Disability Index (HAQ-DI) was consisted of 20 questions regarding the limitations patients experience in performing daily physical activities [18] which were assisted for all patients in the same visit. Patients were asked how difficult it is to perform an activity on a scale of 0 (without any difficulty) to 3 (unable to do). Patients are also asked whether they need assistance or aids for the activity. X-ray scoring for disease activity using the modified version of the Larsen method [19] was evaluated by rheumatologist and radiologist who assessed joint damage on plain films of the hand or wrist taken at the time of the study. This method analyses plain films of eight proximal interphalangeal joints, two interphalangeal thumb joints, 10 metacarpophalangeal joints, and both wrists. The degree of joint damage was rated as follows: grade 0, normal; grade 1, soft tissue swelling, joint space narrowing, and subchondral osteopenia; grade 2, bone erosion with destruction of < 25% of the joint space; grade 3, 26% to 50% joint space destruction; grade 4, 51% to 75% destruction; and grade 5, > 75% destruction of the joint space. The sum of all scores equaled 110 points.
Serum samples were collected from the patients and controls in order to assay ESM-1. RF IgM was measured by nephelometry, with a level of 20 IU/ml considered positive. ACPA was measured using an enzyme-linked immunosorbent assay with a level of 5U/ml considered positive. ESR and CRP (high sensitive) were measured immediately after blood collection.
2.1. Quantitative Determination of ESM-1 by ELISA Technique.
2.1.1. RF Positive Serum Samples Oreparation
To neutralize the effect of RF antibodies, HeteroBlock (omega Biologicals) reagent was added to the serum samples with positive RF antibody to block and neutralize it (blocking agent) according to manufacturer’s instructions.
Quantification of Human Endothelial Cell-Specific Molecule -1 levels in the sera of all studied subjects was performed by sandwich ELISA technique using a commercially available Enzyme-linked Immunosorbent Assay Kit for Human ESM1 (Cloud-Clone Corp., Wuhan, CHINA). The sera were collected and stored at -20°C to -80°C. All the reagents were brought and the samples were centrifuged again after thawing before the assay. It is recommended that all samples and standards should be assayed in duplicate. 100μL of Standard, Blank, or Sample per well were added. The blank wells were added with Reference Standard & Sample diluent. The plate were covered with sealer. The samples were then incubated for 90 minutes at 37°C. The liquids were then removed from each well, but the sample were not washed. Immediately, 100μL of Biotinylated Detection Ab working solutions were added to each well, then were covered with the Plate sealer. Gently, the plate were tapped to ensure thorough mixing, followed by incubation for 1 hour at 37°C. Each well was aspirated and washed, repeating the process three times. Washing was conducted by filling each well with Wash Buffer (approximately 350μL). After the last wash, remaining Wash Buffer was removed by aspirating or decanting. Add 100μL of HRP Conjugate working solution to each well. Cover with the Plate sealer, followed by incubation for 30 minutes at 37°C. The wash process was repeated for five times as conducted in step 3. 90μL of substrate solution were added to each well and covered with a new Plate sealer, followed by incubation for about 15 minutes at 37°C. The plate were protected from light. 50μL of Stop Solution were added to each well, which turned the color to yellow immediately. The order to add stop solution should be the same as the substrate solution. The Optical Density (OD value) of each well was determined at once, using a micro-plate reader set to 450 nm. Calculation of the results averaged the duplicate readings for each standard and samples, from which the average zero was subtracted from standard optical density. A standard curve was made by plotting the mean OD value for each standard on the y-axis against the concentration on the x-axis and a premium appropriate curve was drawn over the points on the graph. If the samples were diluted, the concentration calculated from the standard curve was multiplied by the dilution factor. The detection range of test was 15.6-1,000pg/ml with a minimum detectable level less than 6.2pg/ml. This assay has high sensitivity and excellent specificity for detection of ESM1.
2.1.2. Statistical Analysis
SPSS statistical software was used for all statistical analyses (IBM SPSS statistics version 21.0). Numerical data were expressed as mean value ± SD while categorical data presented as numbers and percentage. Variations among groups in clinical & serological data were linked by t-test for normally distributed values. Correlations between variables were estimated by the Pearson correlation. Correlations between categorical data were compared using chi-square analysis. P values < 0.05 were considered statistically significant.
3. RESULTS
Serum levels of ESM-1 were statistically and significantly higher in the patient group than the control group (2652.25±2803.40 vs. 546.74±181.64 pg/ml, P < 0.0001) Fig. (1). Mean level of RF, ACPA, ESR, and CRP were significantly higher in the patients group compared to the control group. As regard gender, age, and BMI, there were no significant difference between both groups Table 1.
| Parameters | RA patients | Healthy controls | P value |
|---|---|---|---|
| Gender (female no. & %) | 75(90.4%) | 18 (90%) | NS* |
| Age(year) | 37.59±8.35 | 36.07±7.71 | NS |
| BMI(kg/m2) | 28.22±6.49 | 29.15±4.64 | NS |
| Disease duration(month) | 38.38±16.77 | ----- | --- |
| ESR(mm/hour) | 40.07± 23.04 | 14.55±7.85 | 0.000 |
| CRP(mg/dl) | 14.98± 19.42 | 2.60± 0.79 | 0.005 |
| RF(IU/dl) | 53.14 ± 57.16 | 9.95± 3.28 | 0.001 |
| ACPA(U/dl) | 24.74± 29.84 | 2.59± 1.23 | 0.001 |
| ESM1(pg./ml) | 2652.25±2803.40 | 546.74±181.64 | 0.000 |
| DAS28 score | 3.69±1.19 | ----------------- | --------- |
| HAQ-DI | 1.36±1.12 | ------------------- | ---------- |
| Modified Larsen score | 1.66±1.04 | -------------------- | ----------- |
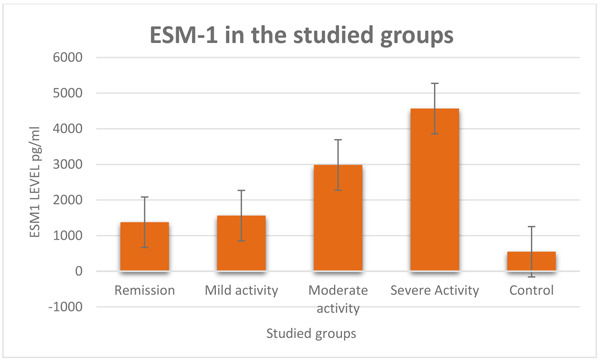
3.1. In the Patient’s Subgroups
In patients with severe disease activity subgroup, compared with patients on remission; serum levels of ESM-1, CRP, ESR, and RF were (P < 0.0001) Fig. (1) were significantly higher (P < 0.0001) Table 2. CRP and RF levels were significantly higher when compared with a patient with moderate disease activity (P < 0.0001 & 0.001 respectively). ESM-1, CRP and RF levels were significantly higher when compared with a patient with mild disease activity (P < 0.0001, < 0.0001 & 0.003 respectively). HAQ-DI and modified Larsen score were significantly higher when compared with other patients’ subgroups.
 |
Remission | Mild Disease Activity | Moderate Disease Activity | Severe Disease Activity | P value |
|---|---|---|---|---|---|
| Age | 36.53±11.03 | 38.87 ±8.11 | 38.18 ±7.87 | 35.87 ±7.09 | NS |
| Gender (female no& %) | 14(93.33%) | 13(86.67%) | 35(92.11%) | 13(86.67%) | NS* |
| BMI | 29.57 ±6.99 | 28.43 ±7.45 | 28.12 ±8.31 | 26.75± 9.22 | NS |
| Disease duration | 39.40±19.67 | 40.93±20.78 | 37.58±14.27 | 36.87±16.70 | NS |
| DAS 28 SCORE | 1.94±0.19 | 2.86±0.28 | 4.05±0.53 | 5.38±0.10 | <0.0001 |
| ESR | 24.33±15.48 | 36.87±34.67 | 43.08±25.40 | 51.40±22.16 | <0.0001 |
| CRP | 3.01±1.35 | 6.80±2.20 | 11.71±7.65 | 43.30±30.61 | <0.0001 |
| RF | 20.44±15.48 | 33.87±34.67 | 50.19±42.78 | 112.55±86.79 | <0.0001 |
| ACPA | 26.25±24.99 | 19.69±24.18 | 21.86±28.05 | 35.56±41.82 | NS |
| ESM1 | 1374.54±556.12 | 1561.47±434.91 | 2989.01±647.84 | 4565.37±437.88 | <0.0001 |
| HAQ-DI | 0.13± 0.35 | 0.40± 0.63 | 1.60± 0.72 | 2.93 ± 0.26 | <0.0001 |
| Modified Larsen score | 0.4 ±0.62 | 1.07 ±0.59 | 1.87± 0.66 | 3.00± 0.53 | <0.0001 |
In patients with moderate disease activity, compared with the patient with remission; ESM-1 was significantly higher (P = 0.040) and ESR, CRP, RF, HAQ-DI, and modified Larsen score were also significantly higher (P = 0.008, < 0.0001, 0.012, 0.0001 &0.0001respectively) but CRP, HAQ-DI, and modified Larsen score were significantly higher (P = 0.018, 0.0001 & 0.0001 respectively) when compared with patient with mild disease activity.
In patients with mild disease activity; serum levels of ESM-1, ESR, CRP, HAG-DI, and modified Larsen score were significantly higher (P = 0.004, 0.034, <0.0001, 0.0001 &0.0001respectively) compared with patients with remission. ACPA levels showed insignificant differences between subgroups.
3.2. Correlations (Pearson Correlation)
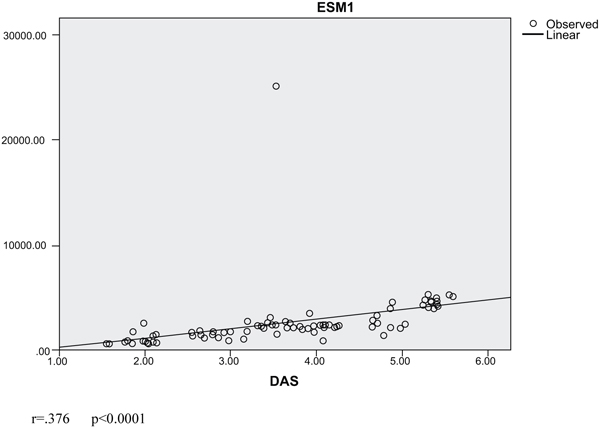
In our cohort, we found that ESM-1 was positively and significantly correlated with DAS-28 score, CRP, and ESR (P = 0.002, 0.035 & < 0.0001, respectively). Also, DAS-28 score was positively and significantly correlated with HAQ-DI and modified Larsen score (P < 0.0001) Table 3. We assessed the linear regression analysis curve estimation between ESM-1 as a dependent variable and DAS-28 which showed a significant correlation (r = .141- P < 0.0001) (Fig. 2).
| Parameters | Pearson Correlation | P value |
|---|---|---|
| DAS- 28 score | 0.342 | 0.002 |
| CRP | 0.232 | 0.035 |
| ESR | 0.423 | 0.000 |
| RF | 0.110 | 0.322 |
| ACPA | -.009 | 0.936 |
| AGE | 0.076 | 0.508 |
| BMI | 0.640 | 0.563 |
| Disease Duration | -.034 | 0.758 |
| HAQ-DI | 0.892 | <0.0001 |
| Modified Larsen score | 0.860 | <0.0001 |
4. DISCUSSION
RA is known by severe angiogenesis, which is required for its pathogenesis [20]. Moreover, the pannus tissue in RA joints displays destructive behavior and attacks and erodes the adjacent cartilage and subchondral bones [17]. ESM-1 serum levels increase in inflammatory disorders [21]. Accordingly, we explored the possible link between RA disease activity and ESM-1. We found that ESM-1 serum levels were higher in patients with RA than the healthy control and is significantly higher in patients with severe disease activity compared with patients with lower disease activity (Table 2). Moreover, its levels were positively correlated with disease activity (DAS-28), HAQ-DI and modified Larsen score (Table 3). This result was partially in line with others who reported the same results in juvenile RA but with lower levels in active disease than those in remission which could be secondary to drug effect or more healthy endothelial in young age and also mostly the absence of vascular disorders, mainly atherosclerosis [22]. It is suggested that serum ESM-1 levels rise in synovial tissue of rheumatoid patients and arouse mainly by adiponectin. Furthermore, ESM-1 can induce angiogenesis, which can elucidate pannus formation [23] and was detected in affected inflamed joints and its level was correlated with inflammation magnitude [23]. We reported a positive correlation between ESM-1 and ESR and CRP indicated that ESM-1 is linked to the inflammatory state in RA but another study failed to report such correlation [22]. Others reported that ESM-1 was elevated and correlated with ESR and CRP in Behcet’s disease [14], SLE [24], SS [25] & IBD [13]. The production of ESM-1 is controlled by pro-inflammatory factors like Tumor Necrosis Factor alpha (TNF-α) and also by pro-angiogenic agents, including VEGF and FGF-2 [26, 27] which may explain the high cardiovascular risk in these patients than the others and ESM-1 can be considered as a marker for vascular endothelial dysfunction [28]. In patients subgroups, we found significantly high levels of ESR, CRP,RF, and ESM-1 in patients with severe disease activity compared with other subgroups with lower disease activity and remission indicating that ESM-1 may play a role in disease activity. As in our patients, we excluded patients with cardiovascular risk which are a main cause of elevated ESM-1. The association between RF levels and ESM-1 in our patients matched with many previous studies showing that the presence of RF antibodies were related to impaired endothelial function individually from cardiovascular risk factors, in RA patients [29]. However, we detected no link between ESM-1 levels and body mass index while others reported a link between obesity (adipocytes) and ESM-1 mainly through adiponectin, an adipokine, which was implicated in the pathogenesis and progression of RA [23]. We moreover found a positive correlation between ESM-1 and HAQ-DI and modified Larsen score plus a linear correlation between ESM-1 levels and DAS-28 in the studied subgroups (Fig. 2), which supported the link between ESM-1 and RA disease activity and highlighted the possible future use of therapeutic anti- ESM-1 in treatment of RA and management of its activity. Finally, we can concluded that ESM-1could be a biomarker for RA disease activity.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved from the Ethics Committee of the School of Medicine, Mansoura University (R1/16.10.06).
HUMAN AND ANIMAL RIGHTS
No Animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients gave an informed consent when they were enrolled.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


