All published articles of this journal are available on ScienceDirect.
Celecoxib and Diclofenac Plus Omeprazole are Similarly Effective in the Treatment of Arthritis in Patients at High GI Risk in the CONDOR Trial§
Abstract
Objective:
Compare effectiveness of celecoxib versus diclofenac plus omeprazole in improving arthritis signs and symptoms in patients at high gastrointestinal (GI) risk who were enrolled in the CONDOR (Celecoxib vs Omeprazole and Diclofenac in Patients With Osteoarthritis and Rheumatoid Arthritis) trial.
Methods:
CONDOR was a 6-month, prospective, double-blind, triple-dummy, parallel-group, randomized, multicenter trial comparing celecoxib 200 mg twice daily versus diclofenac slow release (SR) 75 mg twice daily plus omeprazole 20 mg daily. Patients were Helicobacter pylori negative, had osteoarthritis (OA) or rheumatoid arthritis (RA), were aged ≥60 years, were with or without a history of gastroduodenal ulceration, or were ≥18 years with previous gastroduodenal ulceration. Patients’ Global Assessment of Arthritis was determined at each study visit.
Results:
A total of 4484 patients were randomized to treatment (2238 celecoxib, 2246 diclofenac SR) and included in the intention-to-treat analyses. Least squares mean (LSM) (standard error [SE]) for Patients’ Global Assessment of Arthritis was 3.219 (0.017) and 3.221 (0.017) at baseline for celecoxib and diclofenac SR (p=0.90). Improvement in both groups was similar in months 2, 4, and 6; at month 1 the LSM (SE) was 2.647 (0.017) and 2.586 (0.017) for celecoxib and diclofenac (p=0.0025). LSM difference (SE) from baseline to final visit demonstrated an improvement of 0.75 (0.02) in celecoxib-treated patients and 0.77 (0.02) in diclofenac SR-treated patients (p=0.42).
Conclusions:
Celecoxib and diclofenac plus omeprazole were shown to have similar efficacy in patients with OA and/or RA at increased GI risk who were enrolled in the CONDOR trial.
Trial Registry:
Trial was registered under ClinicalTrials.gov identifier NCT00141102.
INTRODUCTION
Treatment goals in patients with arthritis focus on reducing pain and inflammation, and on improving functional activity [1, 2]. Nonsteroidal anti-inflammatory drugs (NSAIDs), including nonselective NSAIDs and cyclooxygenase-2 (COX-2) selective NSAIDs, are used widely in the management of pain and inflammation associated with osteoarthritis (OA) and rheumatoid arthritis (RA) [3].
Although the efficacy of nonselective NSAIDs in arthritis is well established, use of these agents is associated with numerous adverse events, including upper and lower gastrointestinal (GI) toxicity [4-9]. All prescription NSAIDs have the same warning for serious GI events from the US Food and Drug Administration [10]. Physicians are faced with a difficult clinical decision in selecting the best treatment option for individual patients, particularly those at high risk of GI events, that balances effectiveness against arthritis signs and symptoms alongside the potential for adverse events.
COX-2 selective NSAIDs were developed to potentially reduce the GI adverse events caused by nonselective NSAIDs [11] while retaining similar efficacy [12]; several lines of evidence suggest that use of COX-2 selective NSAIDs may confer a reduced GI risk, particularly in the lower GI tract [13-15]. These observations have led to the publication of clinical guidelines that recommend the use of a nonselective NSAID plus a proton-pump inhibitor (PPI) or a COX-2 selective NSAID in patients with arthritis at risk of GI adverse events [1, 16-20].
However, while there are a limited number of studies comparing the efficacy and safety of celecoxib versus diclofenac [7, 21-25], there are very few studies in patients at high risk of GI adverse events. The CONDOR (Celecoxib versus Omeprazole and Diclofenac in Patients With Osteoarthritis and Rheumatoid Arthritis) trial, was the first prospective, large-scale clinical trial that showed that the risk of clinical outcomes across the entire GI tract was significantly reduced in patients with arthritis at high GI risk treated with celecoxib compared with those treated with diclofenac slow release (SR) plus omeprazole [7]. Treatment efficacy of celecoxib versus diclofenac SR plus omeprazole was also determined as a secondary outcome [7]. The aim of the present analysis was therefore to compare the effectiveness of celecoxib versus diclofenac plus omeprazole in improving arthritis signs and symptoms in patients at high GI risk who were enrolled in CONDOR.
METHODOLOGY
Patients and Study Design
CONDOR was a 6-month, prospective, double-blind, triple-dummy, parallel-group randomized trial conducted across 32 countries or territories. Patients with OA and/or RA with an increased risk of GI events were randomized 1:1 to receive either celecoxib 200 mg twice daily (bid) or diclofenac SR 75 mg bid plus omeprazole 20 mg once daily (qd) for 6 months. The detailed inclusion and exclusion criteria, study design, and methods have been published previously [7] and are briefly discussed.
Patients with a clinical diagnosis of OA or RA were eligible for study entry if they were aged ≥60 years, with or without a history of gastroduodenal ulceration, or were aged ≥18 years and had documented evidence of gastroduodenal ulceration 90 days or more before screening. Patients also had to test negative for Helicobacter pylori at screening or have confirmed eradication of infection at a rescreening visit. Patients were excluded if they had a GI hemorrhage or active gastroduodenal ulceration within 90 days of screening and if they were concomitantly using antiplatelet (including aspirin) or anticoagulant therapy. Eligible patients were randomized to treatment at the baseline study visit and returned to the clinic at months 1, 2, 4, and 6 for assessments.
The study was conducted in accordance with Good Clinical Practice and the protocol was approved by local institutional review boards. All patients provided written informed consent.
Efficacy Assessments
The primary efficacy assessment was the Patients’ Global Assessment of Arthritis; this efficacy assessment provides good test-retest reliability in arthritis [26]. The Patients’ Global Assessment of Arthritis was determined at each study visit (screening, baseline, and months 1, 2, 4, and 6) by asking the following question: “Considering all the ways the OA or RA affects you, how are you doing today?” Patients rated their arthritis on a 5-point Likert scale, where 1 was very good and 5 very poor.
Statistical Analyses
All analyses in the study were based on the intention-to-treat (ITT) population (unless otherwise stated); the ITT population included all patients who were randomized to treatment. Baseline demographics and characteristics were summarized using descriptive statistics. Treatment comparisons based on the Patients’ Global Assessment of Arthritis were analyzed using a general linear model, including geographic region and history of gastroduodenal ulceration as fixed effects and Patients’ Global Assessment of Arthritis at baseline as a covariate. A last observation carried forward approach was applied to the final visit. Responses were also summarized by category and compared between treatment groups using a Cochran-Mantel-Haenszel (CMH) method. A p value <0.05 was considered statistically significant.
RESULTS
Patients
A total of 4484 patients were included in the ITT population (2238 celecoxib, 2246 diclofenac plus omeprazole). 1730 (77.3%) patients treated with celecoxib and 1621 (72.2%) patients treated with diclofenac SR plus omeprazole completed the study. Compliance with study medication was similar in both treatment groups (0.99 [0.03] celecoxib, 0.99 [0.05] diclofenac plus omeprazole).
The mean age of the study population was 65 years and the majority of patients were female (82%). There were no major differences between treatment groups with respect to demographic or baseline characteristics (Table 1). The majority of patients had a diagnosis of OA (84% [3774/4484] of patients versus 16% [710/4484] of patients with RA). The mean disease duration of OA was 7.6 years and 7.8 years in the celecoxib and diclofenac plus omeprazole groups, respectively. Mean disease duration of RA was 10.2 years for patients treated with celecoxib and 9.9 years for those treated with diclofenac plus omeprazole.
Baseline Demographics and Characteristics in Patients with Arthritis Enrolled in the CONDOR Trial (ITT Population)
| Celecoxib 200 mg bid | Diclofenac SR 75 mg bid Plus Omeprazole 20 mg qd | |
|---|---|---|
| Total n=2238 |
Total n=2246 |
|
| Age, Years, n (%) | ||
| <55 | 176 (7.9) | 164 (7.3) |
| 55-59 | 122 (5.5) | 113 (5.0) |
| 60-64 | 721 (32.2) | 742 (33.0) |
| 65-69 | 623 (27.8) | 618 (27.5) |
| 70-74 | 361 (16.1) | 390 (17.4) |
| ≥75 | 235 (10.5) | 219 (9.8) |
| Mean (SD) | 65.2 (7.8) | 65.3 (7.6) |
| Range | 26-89 | 25-93 |
| Sex, n (%) | ||
| Male | 390 (17.4) | 424 (18.9) |
| Female | 1848 (82.6) | 1822 (81.1) |
| Race, n (%) | ||
| White | 1238 (55.3) | 1212 (54.0) |
| Black | 49 (2.2) | 57 (2.5) |
| Asian | 299 (13.4) | 311 (13.8) |
| Hispanic | 462 (20.6) | 464 (20.7) |
| Other | 190 (8.5) | 202 (9.0) |
| Weight (kg), n (%) | 2231 (99.7) | 2242 (99.8) |
| Mean (SD) | 72.5 (15.2) | 72.9 (14.8) |
| Range | 37.5-186.0 | 37.9-150.0 |
| Height (cm), n (%) | 2232 (99.7) | 2242 (99.8) |
| Mean (SD) | 159.1 (9.3) | 159.7 (9.4) |
| Range | 130.0-199.0 | 130.0-192.0 |
| Primary Diagnosis, n (%) | ||
| OA | 1884 (84.2) | 1890 (84.1) |
| RA | 354 (15.8) | 356 (15.9) |
| Patients with Any Concomitant Medications, n (%) a | ||
| Total patients | 1871 (84.2) | 1913 (85.5) |
| Most Frequently (>5%) Used | ||
| Amlodipine | 127 (5.7) | 153 (6.8) |
| Atenolol | 110 (4.9) | 128 (5.7) |
| Calcium carbonate | 117 (5.3) | 119 (5.3) |
| Enalapril | 232 (10.4) | 222 (9.9) |
| Hydrochlorothiazide | 198 (8.9) | 199 (8.9) |
| Methotrexate | 187 (8.4) | 197 (8.8) |
| Paracetamol | 395 (17.8) | 395 (17.7) |
| Medical History (Occurring in >2% Patients), n (%) | ||
| Gastroduodenal ulceration | 395 (17.6) | 400 (17.8) |
| Peptic ulcer | 44 (2.0) | 51 (2.3) |
| Gastric ulcer | 133 (5.9) | 150 (6.7) |
| Duodenal ulcer | 228 (10.2) | 212 (9.4) |
| Gastritis | 347 (15.5) | 362 (16.1) |
| Hemorrhoids | 177 (7.9) | 142 (6.3) |
| Anemia | 49 (2.2) | 52 (2.3) |
a Percentages calculated based on safety population (celecoxib, n=2223 and diclofenac, n=2237).
Categorical Summary of Patients’ Global Assessment of Arthritis Scores in Patients Enrolled in the CONDOR Trial Receiving Celecoxib or Diclofenac Plus Omeprazole
| Visit | Category | Celecoxib 200 mg bid n (%) |
Diclofenac SR 75 mg bid Plus Omeprazole 20 mg qd n (%) |
|---|---|---|---|
| Screening | Good/Very good | 280 (12.7) | 285 (12.9) |
| Fair | 1315 (59.7) | 1328 (60.1) | |
| Poor/Very poor | 608 (27.6) | 598 (27.0) | |
| Baseline | Good/Very good | 237 (10.7) | 226 (10.2) |
| Fair | 1286 (58.3) | 1311 (59.2) | |
| Poor/Very poor | 684 (31.0) | 676 (30.5) | |
| Month 1 | Good/Very good | 877 (42.7) | 940 (46.1) |
| Fair | 985 (47.9) | 939 (46.1) | |
| Poor/Very poor | 194 (9.4) | 160 (7.8) | |
| Month 2 | Good/Very good | 1021 (52.0) | 999 (53.3) |
| Fair | 815 (41.5) | 756 (40.3) | |
| Poor/Very poor | 129 (6.6) | 121 (6.4) | |
| Month 4 | Good/Very good | 1037 (56.9) | 1001 (58.1) |
| Fair | 683 (37.5) | 630 (36.6) | |
| Poor/Very poor | 101 (5.5) | 92 (5.3) | |
| Month 6 | Good/Very good | 1214 (57.0) | 1204 (57.6) |
| Fair | 737 (34.6) | 723 (34.6) | |
| Poor/Very poor | 179 (8.4) | 163 (7.8) | |
| Final (LOCF) | Good/Very good | 1250 (56.6) | 1246 (56.3) |
| Fair | 770 (34.9) | 789 (35.7) | |
| Poor/Very poor | 187 (8.5) | 178 (8.0) |
LOCF=last observation carried forward.
Patients’ Global Assessment of Arthritis
Patients’ Global Assessment of Arthritis was similar between treatment groups at baseline, with a least squares mean (LSM) (standard error [SE]) of 3.219 (0.017) for the celecoxib group and 3.221 (0.017) for the diclofenac SR plus omeprazole group (p=0.90) (Fig. 1). Improvement in both treatment arms was similar in months 2, 4, and 6; at month 1 the LSM (SE) was 2.647 (0.017) and 2.586 (0.017) for celecoxib and diclofenac SR, respectively (p=0.0025). The LSM (SE) of Patients’ Global Assessment of Arthritis at final visit or early termination (last observation carried forward) was 2.474 (0.02) in the celecoxib group and 2.455 (0.02) in the diclofenac group.
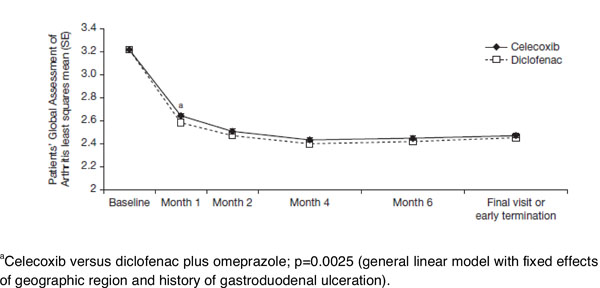
Improvements in the least squares mean of the Patients’ Global Assessment of Arthritis from baseline to final visit or early termination in patients enrolled in the CONDOR study receiving celecoxib or diclofenac plus omeprazole
The LSM difference (SE) from baseline to last observation carried forward demonstrated an improvement of 0.75 (0.02) in celecoxib-treated patients and 0.77 (0.02) in diclofenac plus omeprazole–treated patients (p=0.42). These findings were reflected in the categorical summary of Patients’ Global Assessment of Arthritis score; compared with baseline, more patients scored their arthritis as good or very good following 6 months of treatment with celecoxib or diclofenac plus omeprazole (Table 2). There was no significant difference in the categorical summary of Patients’ Global Assessment of Arthritis score at the final visit between treatment groups using CMH (p=0.9053).
DISCUSSION
When considering appropriate NSAID treatment strategies for individuals with arthritis, physicians must balance the efficacy alongside safety of the NSAIDs. This secondary analysis of data from the CONDOR trial demonstrates that celecoxib and diclofenac SR plus omeprazole have comparable efficacy in patients with OA and RA who are at increased GI risk. Patients in both treatment groups experienced an improvement in arthritis during the 6 months of the study as evidenced by a reduction in scores on the Patients’ Global Assessment of Arthritis. Compared with baseline, more patients rated their arthritis as good or very good following 6 months of treatment with either intervention.
This study has shown that celecoxib and the non-selective NSAID diclofenac are equally efficacious in the treatment of OA and RA. These findings further support previous studies and meta-analyses in which celecoxib was consistently found to have similar efficacy to nonselective NSAIDs, including diclofenac and naproxen, in patients with OA or RA [21, 24, 27-30].
It should be noted that the dose of celecoxib (200 mg bid) studied in the CONDOR trial is the maximum licensed dose for the treatment of OA and RA [31], and, as such, may not accurately reflect the dose commonly used in current clinical practice. However, earlier studies assessing escalating doses of celecoxib indicate that 100-mg bid and 200-mg bid doses of celecoxib are similarly efficacious to one another and to nonselective NSAIDs in patients with OA or RA [27, 29].
Data from the CONDOR trial have demonstrated that, in patients at high GI risk, celecoxib is as efficacious as diclofenac SR plus omeprazole in improving the signs and symptoms of arthritis but it is associated with significantly fewer GI events. COX-2 selective NSAIDs and nonselective NSAIDs remain an important component of the therapeutic armamentarium for arthritis, provided the relative benefits and risks are assessed in individual patients.
AUTHOR’S CONTRIBUTIONS
H.L. Kellner – conduct of study, analysis and interpretation of data, critical revision/drafting of the manuscript, final approval to submit.
C. Li – statistical analysis and interpretation, critical revision/drafting of the manuscript, final approval to submit.
M.N. Essex – analysis and interpretation of data, critical revision/drafting of the manuscript, final approval to submit.
CONFLICT OF INTEREST
H.L. Kellner – consultant/advisor for Pfizer and member of Pfizer’s speakers’ bureau.
C. Li – Pfizer Inc. full-time employee and shareholder.
M.N. Essex – Pfizer Inc. full-time employee and shareholder.
ACKNOWLEDGEMENTS
The study was sponsored by Pfizer Inc, New York, NY, USA. Editorial support was provided by K. Bradford, PhD, and C. Campbell, PhD, of PAREXEL, UK and was funded by Pfizer Inc.


