All published articles of this journal are available on ScienceDirect.
Efficacy and Safety of Rituximab in Biologic-Naive Patients with Rheumatoid Arthritis vs Anti-Tnf Therapy Failure
Abstract
Objectives:
Our aim was to compare an AntiCD20 therapy (rituximab) for rheumatoid arthritis in two patient populations (Group 1), anti-TNFα naïve patients and inadequate responders to Anti-TNFα therapy (Group 2).
Methods:
We analyzed the efficacy of the drug Rituximab (RTX) in RA patients who failed methotrexate (MTX) or had a relative or absolute contraindication to receive anti-TNFα therapy.
Results:
25 patients were identified according to the above criteria and followed up for a mean period of 6 months. Thirteen patients were biologic naïve and twelve patients had already failed anti-TNFα therapy. Group 1 used 2> DMARDs (32% vs 20%, p<0.005), group 2 had more years of disease progression (5±1.89 v s4.10±3.92, p<0.001). The remission as measured by the DAS28 reached faster in group 1 (1.25±0.12 vs 2.15±1.64, p<0,001). Severe infections especially by herpes viruses were more frequent in group 2.
Conclusions:
Comparing clinical improvement in both groups the decrease of acute phase reactants and the clinical remission measured by DAS28 was reached in both groups, however it was reached more belatedly in group 2 (at 6 months), this is due to the fact that they have more years of the disease evolution and a higher HAQ.
INTRODUCTION
Following the discovery of the Rheumatoid Factor (RF) in the decade of the 40’s by Waaler and Rose, RA was accepted as a systemic disease capable of developing multiple antibodies directed against the synovial membrane where the B cells had a leading role [1], as it was implicated in the regulation of the immune system, since beyond the production of antibodies, they are antigen presenting cells that interact with other mononuclear cells and contribute directly in the inflammatory channels; however in 1957 a fact was documented suggesting that this fact was not necessarily true, by proving the concurrence of RA with the agammaglobulinemia produced by inactive B lymphocytes in various patients and it was thought that the presence of B cells was not necessary for the development of RA [2, 3].
As of the decade of the 60-70’s and based upon the predominance of T lymphocytes (TLP) in the synovia of patients with RA [4], these cells obtained a leading role which was boosted after evidencing that CD4+T cells activated by antigens, stimulate synovial monocytes, macrophages and fibroblasts in order to produce cytokines IL-1, IL-6 and anti-TNFα. Although, the development of arthritis by collagen experimental models in mice depends upon the presence of B cell, since animals deficient of these cells are resistant [5].
The synovial membrane in patients affected by RA is characterized by hyperplasia, increase of vascularity and presence of an infiltration of inflammatory cells, particularly CD4+T cells, which are the principal component of the adaptive immune response. In genetic studies, RA is strongly associated with the presence of class II molecules of the histocompatibility principal complex, especially of the HLA-DRB1*0404 and DRB1*0401 alleles [6]. The principal function of these molecules is to present antigenic peptides toCD4+T cells, which suggests that RA is caused by arthritogenic antigens which are not yet well identified. These antigens could be exogenous proteins, e.g. viral or endogenous proteins, such as citrullinated proteins, glycoprotein 39 of human cartilage, HSP and union to the heavy chain proteins [7-9].
In spite of the arrival of new biological therapies, particularly Anti-TNFα therapies, a rate of more than 30% of non-responders is described, moreover having a higher rate of efficacy or tolerance loss having more than 3 years receiving anti-TNFα[10], recommending the use of other molecules such as Anti-CD20, Anti-IL6 or co-stimulation inhibitors (CTLA-4) in case of biological failure; notwithstanding the rates of efficacy and remission are very low (ACR 70 <35%) and the rate of adverse events increases as explained by the years of application and the accumulation of immunosuppressive therapy, for this reason the following study was designed [11,12].
THE OBJECTIVE OF THE STUDY
Determine the efficacy and safety of the Rituximab anti-B cell (AntiCD20) drug in patients with rheumatoid arthritis who have failed therapy with synthetic DMARDs, compared with patients in whom the anti-TNF- biological therapy failed.
PATIENTS AND METHODS
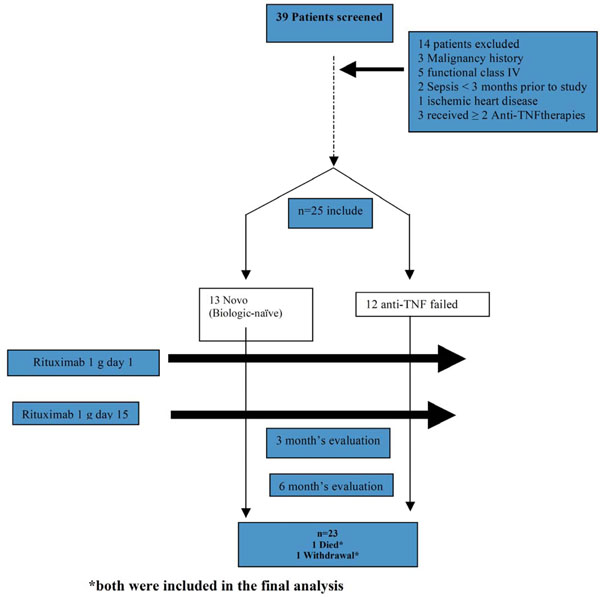
The type of the study is prospective longitudinal where the selection was done by the aliquot method (patients that consulted a specialized clinic in rheumatology and fulfilled all the selection criteria). 25 patients diagnosed with rheumatoid arthritis were included according to the criteria of the American College of Rheumatology (ACR 1987). The data of patients were submitted to HAQ and DAS28 surveys, visual analogous scale (VAS), also X-ray of hands and feet with fine grain industrial plaque, routine laboratory: Complete blood count, erythrocyte sedimentation rate (ESR), “C” reactive protein (CRP), blood chemistry, urinalysis, immune-rheumatologic profile (ANA, rheumatoid factor, double stranded Anti-DNA, Anti-CCP). Clinical and radiological evaluations were executed at 3 and 6 months after starting the study.
The inclusion criteria were the following patients: 18 years of age or older, with RA according to ACR 1987 criteria, in functional I-II class and in activity with DAS28 >2.6 and increased acute phase reactants CRP < 6mg/dl or ESR >20 mm). We proceeded to divide them in two (2) groups, the first with inadequate response to treatment with leflunomide and/or MTX one average dose of 20 mg/d or 15 mg/week respectively, at least during 12 weeks at a stable dose the last 4 weeks before screening, and the second group with inadequate response to Anti-TNFa therapy during at least 24 weeks of continuous treatment. The use of disease modifying drugs (DMARD) and the concomitant use of glucocorticoids were allowed, a stable dose not higher than 10mg daily with prednisone or its equivalent, in both groups a dose of 1 gram of rituximab day 1 and at day 15 was applied.
Pregnancy patients, patients with a previous diagnosis of overlap syndrome, SLE and/or mixed connective tissue disease, recent severe infection (< of 1 year), history of malignant neoplasm disease or in remission from it for less than 2, were excluded.
At the beginning of the study and at month 6, hand and feet X-rays were performed and they were analyzed by the Van der HeijdeSharp modified method. Progression or severity of the disease was defined, if in the radiological control study erosions appeared in patients who did not have them at the beginning, or if there was an increment in the grade of erosion in those who did not have it at the beginning of the study (Fig. 1).
Enrollment and Outcomes.
DAS28 score variations at 0, 3 andd 6 months.
HAQ score differences at 0, 3 and 6 months.
Statistical Analysis
As patients entered the study, data obtained were stored in a data base contained within the statistical program SPSS Version 10.00. After completing simple recollection we proceeded to perform the quantitative analysis of the data, for which the descriptive analytical methods were applied such as: averages, percentages and standard deviations, rate of prevalence, among others, such as risk association measures with the odds ratio and the exact Fisher test.
RESULTS
25 patients were included (n=25) with an RA diagnosis who had therapy failure with non-biological DMARD and biological DMARDS, they were distributed into 2 groups, the first with 13 patients with a DMARD failure and who never received biological treatment (Biologics naïve) and the second group with 12 patients who failed the Anti-TNFα therapy (Table 1). The average age was 52.32± 6.09, more than 90% were feminine and in average, the disease’s duration was higher in the group of the Anti-TNFα failure (10.04±3.92). 75% of the patients in group 1 received methotrexate in comparison with 82% of group 2, less than 8% in both groups received leflunomide, 23.4% of patients received hydroxychloroquine in group 1 and only 9% in group 2 (Table 1).
Patients Baseline Characteristics
| Age (Years ± SD) | Group 1 n=13 (Biologic-Naïve) 50.32 ±4.57 | Group 2 n=12 (Anti-TNFα Failed) 52±6.09 | P Value p=0.08 |
|---|---|---|---|
|
|
|||
| Sex | |||
| Male | 1 (4%) | 1 (4%) | |
| Female | 12 (48%) | 11 (44%) | |
|
|
|||
| DMARD numbers | |||
| One (1) used | 5 (20%) | 7 (28%) | |
| 2 ≥ more frequently used | 8 (32%) | 5 (20%) | p<0.005 |
|
|
|||
| Duration of the disease years ± SD | 5±1.89 | 10.04 ± 3.92 | p<0.001 |
|
|
|||
| ESR baseline (M±SD) | 47.39 ± 7.69 | 35±5.12 | p<0.001 |
|
|
|||
| CRP baseline mg/dl: (M±SD) | 12.25±2.68 | 9±1.08 | p<0.001 |
|
|
|||
| DAS28 baseline (M±SD) | 4.93±0.37 | 3.78±0.12 | p=0.34 |
|
|
|||
| HAQ baseline: (M±SD) | 2.33±0,21 | 2.55±0.11 | p=0.08 |
|
|
|||
| Rheumatoid Factor positive (%) | 11 (84.6%) | 12 (100%) | p=0.81 |
|
|
|||
| Anti CCP positive >25 Ui/ml | 8 (61.5%) | 9 (75%) | p=0.1 |
SD: standard deviation. DAS: Disease Activity Score. HAQ: Health Assessment Questionnaire. ESR: erythrocyte sedimentation rate. CPR: “C” reactive protein, RF-Test: Screening Test for Rheumatoid Factor. Anti-CCP: Antibodies against citrullinated peptides. DMARD: disease-modifying anti-rheumatic drugs.
Changes of Baseline DAS28, HAQ, VSG and CRP at 6 Months
| Group 1 | Group 2 | P Value | |||
|---|---|---|---|---|---|
| Baseline | 6 m | Baseline | 6m | ||
| ESR mm | 47,39 ± 7,69 | 12±1.23 | 35±5.12 | 15±2.89 | 0.081 |
| CRP mg/dl | 12,25±2,68 | 4±0.89 | 9±1.08 | 6±0.26 | 0.164 |
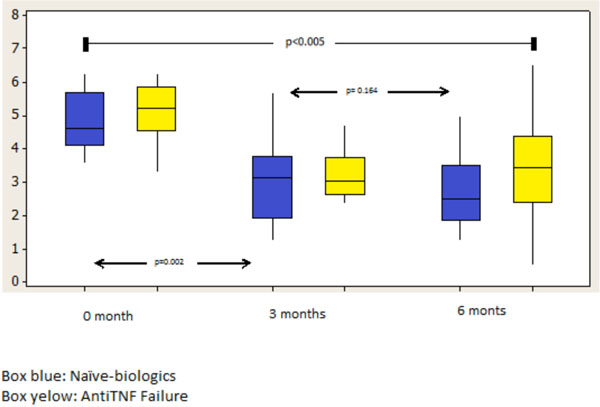
| DAS 28 | 4,93±0,37 | 1.25±0.12 | 3.78±0.12 | 2.15±1.64 | <0.001 |
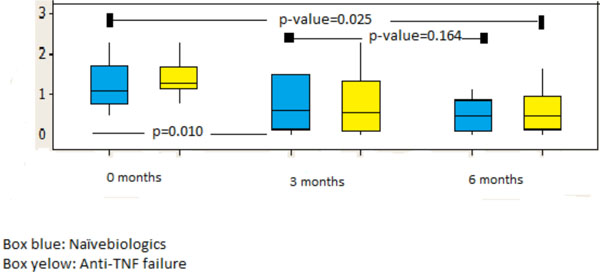
| HAQ* | 2.33±0,21 | 0.758±0.11 | 2.55±0.11 | 1.18±0.47 | <0.001 |
* Scores for the Stanford Health Assessment Questionnaire (HAQ) can range from 0 to 3, with higher scores indicating greater disease activity.
Adverse Events
| Adverse Events | Group 1 | Group 2 | P Value |
|---|---|---|---|
|
|
|||
| Deaths | 0 | 1* | 1.0 |
|
|
|||
| Serious infections | 0 | 2 | |
| Virus Herpes Zoster infection | 0 | 2 | |
| Ramsay Hunt syndrome | 0 | 2 | <0.001 |
| Pneumonias | 0 | 1 | 1.0 |
| Acute sinusitis | 1 | 0 | 1.0 |
|
|
|||
| Other Adverse Events | |||
|
|
|||
| Flu-Like | 1 | 1 | 1.0 |
| Hematologic toxicity | 1 | 1 | 1.0 |
| Hypersensitivity reaction | 1 | 0 | 0.34 |
* Patient died during an abdominal chirurgic intervention.
Even though in group 1 there were fewer patients with positive rheumatoid factor, they received rituximab (the guidelines recommended or suggested their use only in seropositive patients for RF). The accumulated dose for the glucocorticoids was 3.57±1.48 in group 1 while in group 2 it was 7.85±2.57.
The 6 months cohort, group 1 had a significant difference in HAQ changes with a p<0.001 and a relative risk of DAS28 2.85, 95% confidence interval (CI) 2.08 to 3.91 compared with the group 2 (Table 2). In the study no anti-TNFα antibodies anti chimeric (HACA) were requested, even though 7 patients in group 2 received infliximab, 4 received adalimumab and only 1 patient etanercept.
There was no progression of the disease from the radiological point of view for both groups, and the proportion of patients with changes from the baseline to the cutoff date at 6 months was less than -0.5 and without deterioration of punctuation of the erosion (more than 1 in any joint according to Van der Heijde modified Sharp’s Score).
Declines of DAS28 were observed in both groups at 3 and 6 months, even though the greatest decline was observed in the cohort in month 6 in group 1 with a p<0.001 in comparison to group 2 (Fig. 2). Similarly, while evaluating HAQ at 3 and 6 months in group 1 there was a decline below 1.00 (HAQ), maintaining the values above 1.00 (group 2 HAQ 6m) for those who received Anti-TNF therapy previously (Fig. 3).
Adverse Events
Within the severe adverse events mostly observed was infection by herpes Zoster (2 patients), both occurred in patients older than 55 years of age, who had already received 2 DMRDs and Anti-TNF therapy, both were in group 2. Within the most common post transfusion reactions there were 4 flu-like which improved with the use of common antipyretics (acetaminophen), no lupus-like, no modified psoriasis were observed, 2 cases of hematological toxicity were evidenced: one case of bicitopenia and one case of non-feverous neutropenia, both referred to a hematological specialist which were catalogued as transitory, one allergic reaction (cutaneous toxicity: urticarial) which was resolved with anti-histaminic of 3rd generation (Table 3). One patient in group 2 died during a surgical intervention of cholecystectomy, the cause was a heart attack during surgery.
DISCUSSION
The treatment of rheumatoid arthritis has changed radically in the last decade, the use of the pyramidal treatment for RA already seems as an anachronism, while treatments were initiated in a staggered manner with nonsteroidal anti-inflammatory (NSAIDs), leaving DMARDs as a second option [13,14], which made many patients develop a disabling disease in a short time [15]. Currently, the international guidelines or consensus recommend the use of DMARDs in early RA, and should there be failure to MTX in 3 to 6 months the patients should be transferred to biological therapy with Anti-TNFa, this is due to the fact that in clinical studies in the decade of the nineties, the chief cytokine or pivot cytokine in rheumatoid arthritis was considered to be the TNFa [16-20], however in recent years other types of anti-cytokines have developed (Anti IL-1, Anti IL-6 y Anti IL-21/23), in response to a group of patients who had failed the anti-TNFa therapy [21-26].
We have evaluated the efficacy and safety of rituximab in patients with active RA and with an inadequate response to anti-TNFa therapy. We also compared the use in patients with established rheumatoid arthritis (not early arthritis) and failure to DMARDs, in both cases there was a clinical improvement and the radiographic progression of the disease was avoided, even though a major remission was observed in the group with a lesser progression of the disease, furthermore the HAQ punctuation finalized below 1.00 in group 1, which was reflected in a better functional capability.
Serious adverse events were more frequent in the group of the anti-TNFa failure, and this is due to the fact that it is a group of patients with older age, with a longer time of disease and with an accumulation of immune suppressor drugs, which make them more susceptible to severe infections.
Comparing clinical improvement in both groups the decrease of acute phase reactants and the clinical remission measured by DAS28 was reached in both groups, however it was reached more belatedly in group 2 (at 6 months), this is due to the fact that they have more years of the disease evolution and a higher HAQ. Perhaps instead of using this drug in patients who failed anti-TNFatherapy, it can be used in patients with early arthritis [27] or simply low dose, for example once a year, to prevent adverse events which have increased as demonstrated by studies Dougados in patients who already had good control or low disease activity [28].
This paper describes a group of patients improved with the use of anti-CD20 therapy, this is a well-known phenomenon, however clinical improvement, adverse events and radiographic progression were better in the group with the lowest time of evolution of the disease and less accumulation of drugs, so you might see use incase synthetic DMARDs fail.
Medical literature describes that there is a failure rate to anti-TNFα therapy ranging from 30-35%, there is even a group of patients who do not respond to methotrexate and who have a relative or absolute contraindication to use Anti-TNFα antagonist as are patients with previous TB infection, malignancy, recent ischemic heart disease or class functional III-IV heart failure [29, 30].
We know that the limitation of the study is the low number of patients, however it is the first study in which we speak of an option(safe and effective) in patients who cannot receive AntiTNF therapy. In our country (and sure in many other), government institutions approved this drug only in RA when AntiTNFatherapy fails.
It is our hope that new tools will be developed that may aid us in choosing the appropriate medication for each type of patient, as for example the creation of a test for immune phenotype or immune chip where we could obtain some information about the type of cellularity or predominant cytokine (cytokine pivot) that is affecting a specific patient [31,32].
AUTHORS' CONTRIBUTIONS
MM participated in the design of the study and included patients of his daily practice; MR included patients of his daily practice and examined X-ray; IO participated in the design of the study and included patients of her daily practice; ZT participated in the design of the study, performed the statistical analysis and included patients of his daily practice; LAG wrote the manuscript, conceived the study and participated in its design and coordination. All authors read and approved the final manuscript.
ABBREVIATIONS
| MM | = MarialinaMarín |
| MR | = Marco Rivera |
| ZT | = Zair Tovar |
| IO | = Ibell Oropeza |
| LAG | = Luis Arturo Gutiérrez |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Lic. Isabel Moreno Bilancieri.
DISCLOSURE
Roche Laboratory provides financial support for the radiological and laboratory studies. Biological Drug or synthetic DMARD was provided by the state through IVSS (Instituto Venezolano de los Seguros Sociales), none of the authors received financial support, nor work for Roche or a government enterprise.


