All published articles of this journal are available on ScienceDirect.
Symptom-Modifying Effect of Chondroitin Sulfate in Knee Osteoarthritis: A Meta-Analysis of Randomized Placebo-Controlled Trials Performed with Structum®
Abstract
Objective:
To perform a meta-analysis of randomized double-blind placebo-controlled clinical trials to assess the efficacy of a specific chondroitin sulfate preparation (Structum®, Laboratoires Pierre Fabre, Castres; France) as a symptom-modifying drug in osteoarthritis (OA) of the knee.
Methods:
A Medline search was conducted up to October 2010 and two articles reporting two trials were identified; one additional trial was identified through contacting the producer of Structum®. There was no evidence of heterogeneity across the trials and results were pooled using a fixed effects model.
Results:
Pooled results demonstrated a modest, but significant effect of Structum® (1 g daily) over placebo on the reduction of pain during activity following a treatment period of 3-6 months of 6 mm (95% confidence interval (CI) -9.50, -1.72, p=0.005) on the visual analog scale (VAS) and a reduction in the algo-functional Index (AFI) by a weighted mean difference of -0.73 (95% CI -1.28 to -0.18, p=0.01). In addition, the pooled analysis demonstrated a statistically significant increase in OMERACT-OARSI responders in the Structum®-treated patients by 20% (RR of 1.20 (95% CI 1.06 to 1.36, p=0.003)), compared to placebo.
Conclusion:
These results demonstrate that this chondroitin sulfate preparation (Structum®) is effective on symptoms in patients with OA of the knee compared to placebo, and may therefore have a role in the management of patients with knee osteoarthritis of Kellgren-Lawrence grades II and III.
INTRODUCTION
A recently published meta-analysis [1] concluded that the use of pharmaceutically produced, prescription branded chondroitin sulfate, glucosamine sulfate and their combination should be discouraged for the management of patients with symptomatic OA of the knee. This conclusion was based on a minimal clinically important difference in pain reduction between active preparations and placebo (-9 mm on the 100 mm VAS and triggered a highly controversial discussion regarding the selection of published clinical trials for conducting a meaningful meta-analysis [2-6]. In the wake of this discussion the origin of the active ingredients in the prescribed preparations was considered to be the most important factor ensuring quality, and thus safety and efficacy, in particular for chondroitin sulfate, due to its extraction from different sources.
Sodium chondroitin sulfate is available in a number of oral preparations produced by various manufacturers. It is typically extracted from bovine and porcine tracheal cartilage or from fish or avian cartilage [7]. Structum® is a chondroitin sulfate preparation of avian origin and is internationally approved since 1993. Although its safety has been established beyond any doubt, its efficacy has so far not been demonstrated convincingly in single studies [8, 9].
The primary objective of the present study was thus to assess the clinical efficacy of this preparation by performing a meta-analysis of available clinical trials evaluating Structum® in comparison to placebo at a daily dose of 1g over a treatment period of 3-6 months in patients with OA of the knee.
METHODS
Literature Search
The MEDLINE database (via PUBMED), the Cochrane Controlled Trials Register, and EMBASE were searched as far back as possible, with no language restrictions, up to October 2010 to identify all randomized controlled clinical trials (RCTs) of at least 3 months duration that compared orally administered Structum® to placebo using the following request:
(Chondroitin Sulfates[MeSH Terms] OR Chondroitin Sulfates[TIAB] OR Chondroitin Sulfate[TIAB] OR Chonsurid[TIAB] OR Translagen [TIAB] OR Blutal[TIAB] OR Chondroitin 4-Sulfate[TIAB] OR Chondroitin 4 Sulfate[TIAB] OR Chondroitin Sulfate A[TIAB] OR Chondroitin 6-Sulfate[TIAB] OR Chondroitin 6 Sulfate[TIAB] OR Chondroitin Sulfate C[TIAB] OR Structum[TIAB]) AND (Osteoarthritis[MeSH Terms] OR Osteoarthritis[TIAB] OR Osteoarthritides[TIAB] OR Osteoarthrosis[TIAB] OR Osteoarthroses[TIAB] OR Degenerative Arthritides[TIAB] OR Degenerative Arthritis[TIAB] OR Osteoarthrosis Deformans[TIAB]) AND (("clinical"[TIAB] AND "trial"[TIAB]) OR "clinical trials"[MeSH Terms] OR "clinical trial"[Publication Type] OR "random*"[TIAB] OR "random allocation"[MeSH Terms] OR "therapeutic use"[MeSH Subheading] )
The citations of the retrieved studies, review and meta-analysis articles obtained from the data base searches (snowballing) were also reviewed. In addition, we also searched for unpublished trials and those in progress by contacting the manufacturer of Structum® and by using clinical trials repositories, including that of the National Institute of Health, the National Research Register, Current Controlled Trials, and Trials Central. Abstract of conferences were searched using the ISI proceedings database.
Trials Selection
RCTS of at least three (3) months duration or more that compared orally administered Structum® at a daily dosage of 1 gramme to placebo and reported all outcome measures of interest. The primary outcome was absolute pain intensity (during activity) reported in one of two time windows (three months, more than 3 months). Secondary outcomes were the Lequesne’s algo-functional Index (AFI) [10] or any other function assessment and the rate of responders according to the Outcome Measures of the Rheumatoid Arthritis Clinical Trials and the Osteoarthritis Research Society International (OMERACT-OARSI) criteria [11]. No language restriction was applied.
Data Collection and Quality Assessment
All qualifying studies were assessed for adequate blinding of randomization, completeness of follow up and objectivity of the outcome assessment. Data regarding detailed inclusion criteria, treatment type and duration, duration of follow-up and various outcomes, and safety data were abstracted (as available) from the clinical trial report of each individual study obtained from the manufacturer upon request for data extraction purposes. Data were extracted independently by two reviewers (MC and HS). Disagreements, if any, were resolved by consensus.
To assess studies quality, we evaluated studies for the adequacy of allocation concealment, performance of the analysis according to the intention to treat principle, and blind assessment of the outcome of interest [12]. We used the criteria recommended by Altman and Schulz [13] and Jüni et al. [14] to decide if treatment allocation was adequately concealed or not.
Extracted data were entered into a proprietary specific relational database of clinical trial data. All data entries were 100% verified back to the original source (clinical study reports) prior to locking the database for analysis.
For studies with follow-up that continued beyond the randomized phase, efficacy and safety data were to be only extracted during the randomized study period.
Statistical Analysis
The analysis population included all patients in the intent-to-treat (ITT) populations, who received at least one dose of Structum® or placebo.
For continuous variables, treatment effect was analyzed by weighted mean differences (WM or WMD) in the primary analysis and effect size (SMD) in a secondary analysis. The treatment effect for individual trials was calculated as the difference between treated and control groups in the change between end of trial and baseline. For categorical variables, treatment effect was analyzed by relative risk (RR). Between study heterogeneity was analyzed by means of standard chi2 tests (Cochrane), with p<.05 deemed statistically significant. Where no significant statistical heterogeneity was identified, the fixed-effect estimate was used preferentially as the summary measure. We also used the I2 statistic that is independent of the number of studies and quantifies heterogeneity on a scale of 0% to 100%. Very large heterogeneity between studies is usually denoted by I2 values of 75% or more. All meta-analyses were performed using the EasyMA system [15].
RESULTS
Our search yielded 262 citations. After review of titles and abstracts, 39 full-text articles were retrieved for further evaluation and 6 studies were retained for our analysis.
Two additional studies were identified by contacting the manufacturer of Structum® (Laboratoires Pierre Fabre, Castres; France). Of these eight studies, five were not eligible due to use of a non-placebo control design (four) and one due to the use of an inadequate dose of Structum® (2 g/day) [16-20]. A list of the five excluded studies including the reasons for exclusion is provided in Table 1.
Characteristics and References of Excluded Studies
| Study | Treatment | Reason for Exclusion |
|---|---|---|
| Fardellone P; 2009 [16] | Chondroitin sulfate (Structum®) vs Chondroitin sulfate (Chondrosulf®) | not vs placebo |
| Nasonova et al.; 2001 [17] | Chondroitin sulfate (Structum®) + NSAID vs NSAID | open design, not vs placebo |
| Alekseeva et al.; 1999 [18] | Chondroitin sulfate (Structum®) + Ibuprofen vs Ibuprofen | open design, not vs placebo |
| Soroka NF, Chyzh KA; 2002 [19] | Chondroitin sulfate (Structum®) + NSAID vs NSAID | open design, not vs placebo |
| Mazières et al.; 1992 [20] | Chondroitin sulfate (Structum®) 2g/day vs placebo | inadequate dose |
Characteristics of Identified Randomised Placebo-Controlled Trials of Chondroitin Sulfate (Structum®) in Osteoarthritis of the Knee
| Study | Treatment (Daily Dose) | Treatment Duration (Months) | Concealment Adequate | Patient Blinding Adequate | ITT Performed | Patients Randomized (Structum®/Placebo) | Mean Age | % Women | Joint Affected | Outcomes extracted | Funding Source | Jadad Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Knüsel et al. 2000 [21] | Chondroitin sulfate 1g/day (Structum®) | 6 | yes | yes | yes | 145 (72/73) | 61 | 70 | knee | -VAS (pain) -algo-functional index (AFI) -OMERACT-OARSI responder | Laboratoires Pierre Fabre | 5 |
| Mazières et al. 2001 [8] | Chondroitin sulfate 1g/day (Structum®) | 3 | yes | yes | yes | 132 (64/68) | 67 | 74 | knee | -VAS (pain) -algo-functional index (AFI) -OMERAC -OARSI responder | Laboratoires Pierre Fabre | 5 |
| Mazières et al. 2007 [9] | Chondroitin sulfate 1g/day (Structum®) | 6 | yes | yes | yes | 311 (155/156) | 67 | 70 | knee | -VAS (pain) -algo-functional index (AFI) -OMERACT-OARSI responder | Laboratoires Pierre Fabre | 5 |
The characteristics of the three eligible randomized clinical trials with Structum® versus placebo are shown in Table 2. All three studies were sponsored by the pharmaceutical company (Laboratoires Pierre Fabre, Castres, France) manufacturing and marketing Structum® and the clinical study reports were obtained from the manufacturer upon request for data extraction purposes.
A total of 588 patients were enrolled of which 291 were randomly allocated to Structum® and 297 to placebo. The average study size was 98 patients per arm (range 64 to 156 per arm). All trials were double blind and were reported in English language. One study [21] was unpublished and was obtained in the form of a clinical study report from the manufacturer of Structum®. All three trials were conducted either in France and/or in Switzerland and enrolled patients with knee osteoartrithis. The mean age of the participants was similar across the studies (range from 61 to 67 years). The gender spread between the studies also was similar with about 70% female and 30% male patients. Mean baseline pain intensity during activity ranged from 54 - 61 mm (on a 100mm VAS). All three studies were of good quality based on a Jadad score of 5 (range 0-5) [11]. The duration of the studies was at least three months, with two trials running for six months.
Knüsel and colleagues [21] randomized 145 male or female patients suffering from painful femorotibial OA fulfilling American College of Rheumatology (ACR) criteria [22], age between 50 and 80 years, with an algo-functional Index (AFI) between 4 and 13, a pain score during activity of 30 mm or more on the Visual Analogue Scale (VAS), a radiographic grade II or III on the Kellgren-Lawrence Scale and a regular consumption of NSAID for a minimum of three weeks during the last three months before trial begin. The primary outcome measure was the change in the algo-functional index (AFI) following a treatment period with Structum® or placebo over a period of six months. In the ITT analysis the authors reported a non-significant difference in favour of Structum®.
Mazières and colleagues [8] reported results from a randomized trial with 132 patients with femorotibial OA according to ACR criteria, age > 50 years, an algo-functional Index (AFI) between 4 and 11, a pain intensity during activity of at least 30 mm on the VAS, a regular consumption of NSAID for 3 months and a radiographic grade II or III on Kellgren-Lawrence Scale. The primary outcome measure was the change in the algo-functional index (AFI) following a treatment period with Structum® or placebo of three months. Again, a non-significant difference in favour of Structum® was found in the ITT population.
In a second randomized, double-blind, placebo controlled study which included 307 randomized patients, age between 50-80 years, suffering from medial knee OA defined according to ACR criteria for >6 months, with pain during daily activity of >=40 mm on a 100 mm VAS, a Lequesne’s algo-functional index (AFI) score between 6 and 12 and grade II or III of the Kellgren and Lawrence (K/L) radiographic classification on an anterior–posterior radiograph taken in an extended standing position within the previous six months, Mazières and colleagues [9] reported on changes in pain and AFI after a six months treatment with Structum® or placebo. Also this study failed to show a statistically significant efficacy in one of the primary outcome parameters (AFI) although Structum® again showed a better effect than placebo on pain.
Pooled Effect on Pain During Activity, Lequesne’s Algo-Functional Index and OMERACT-OARSI Responder Rate
Data on pain during activity, expressed as change from baseline, were extractable from all three studies. The analysis detected a statistically significant difference in favor of Structum® (Fig. 1) regarding pain during activity, with a reduction of -5.61 mm (95% CI -9.50 to -1.72, p=0.005). No heterogeneity was detected between the trials with I2=0%.
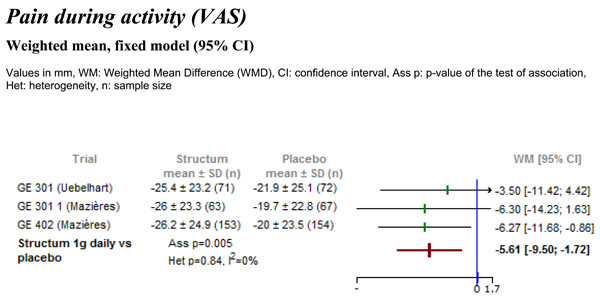
Differences in pain intensity during activity measured on visual analogue scale (VAS) between Structum® interventions and placebo at study end.
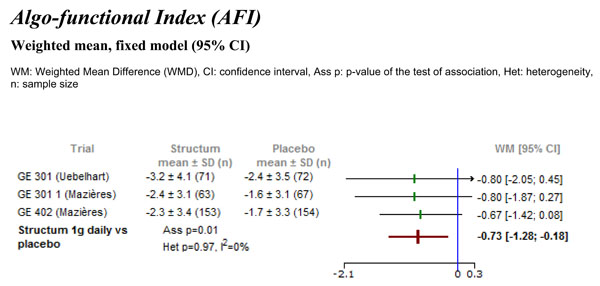
Differences in Lequesne’s algo-functional index (AFI) between Structum® interventions and placebo at study end measured as change from baseline.
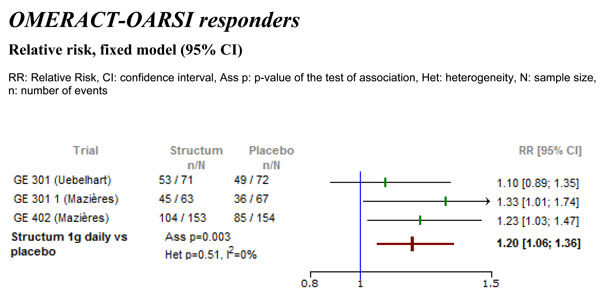
Differences in the percentage of OMERACT-OARSI responders between Structum® interventions and placebo at study end.
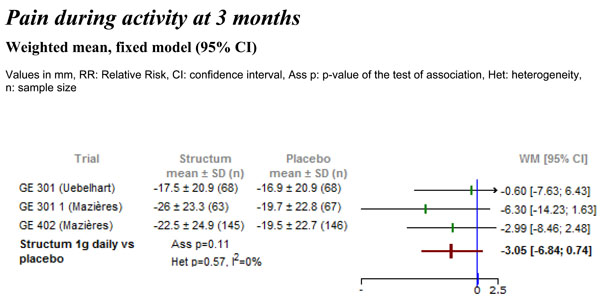
Differences in pain intensity during activity measured on visual analogue scale (VAS) between Structum® interventions and placebo at 3 months.
Data on time-specific, absolute difference of pain intensity during activity vs baseline in the Structum® group were extractable from all three studies. The fixed model analysis (data not shown) detected a statistically significant reduction in absolute pain intensity at study end by -25.9 mm (95% CI -28.7 to -23.2, p<0.001). No heterogeneity was detected between the trials with I2=0%.
All the three studies had extractable data for the Lequesne’s algo-functional index (AFI), expressed as change from baseline. As shown in Fig. (2), the meta-analysis detected a statistically significant difference in favor of Structum® (1 g daily) with a weighted mean difference of -0.73 (95% CI -1.28 to -0.18, p=0.01). No heterogeneity was detected between the results of the three trials with I2=0%.
From one study the percentage of OMERACT-OARSI responders was extractable. For two studies the actual number of OMERACT-OARSI responders was calculated post hoc based on available data in the clinical study reports. The meta-analysis demonstrated a statistically significant difference in favor of Structum® (Fig. 3) with respect to the actual number of OMERACT-OARSI responders, relative risk (RR) of 1.20 (95% CI 1.06 to 1.36, p=0.003) corresponding to an increase under Structum® of the rate of responders by 20%. No heterogeneity was detected with I2=0%.
A time specific analysis of the treatment effect on pain during activity was performed at treatment duration of 3 and 6 months. Three trials reported data at 3 months and two trials had treatment durations of 6 months. At 3 months there was a clear trend towards a decrease in pain during activity (-3 mm vs placebo) observed in all three studies although this effect did not reach statistical significance (Fig. 4). At 6 months (data not shown), based on two studies, a statistically significant effect was demonstrated on pain during activity in favor of Structum® 1g daily with a reduction of -5.4 mm (95% CI -9.86 to -0.22, p=0.02) suggesting a basically linear decrease in pain during activity over time.
DISCUSSION
The findings of this meta-analysis demonstrate that chondroitin sulfate, in the form of Structum® at an oral dose of 1 g/day, has a modest but statistically significant reducing effect of pain intensity, improves functionality (algo-functional index) and increases the actual numbers of OMERACT-OARSI responders in a statistically significant fashion in patients with symptomatic OA of the knee. These results extend the conclusions of previous meta-analyses [23, 24] and support the OARSI recommendations for the management of hip and knee OA regarding the use of chondroitin sulphate as a symptom-modifying drug in patients with knee OA [25].
The present meta-analysis has several strengths. It was based on a systematic literature review, included one unpublished randomized controlled trial and access to the original data source was allowed for data extraction. It included around 300 patients treated with the same dose of 1 g daily of identical product for periods of 3-6 months, and, last but not least, there was no evidence of heterogeneity across the three trials evaluated justifying the use of a fixed effects model. A limitation is that the data on the OMERACT-OARSI responders rates in two studies were calculated post hoc since this outcome had not been included in the original study protocol. Furthermore, the fact that all three trials had the same source of support may be considered a bias.
In many clinical trials on OA of the knee, results are reported as mean and standard deviation of the change, which is not meaningful to most readers since no reference to the clinical relevance of such changes is given routinely. However, incorporating patient perspectives and defining clinically relevant outcomes are of great importance in clinical decision making. For example, in a recently published meta-analysis by Wandel and coauthors [1] on the effects of glucosamine, chondroitin, or placebo in patients with OA of hip or knee, it was concluded that the use of pharmaceutically produced, prescription branded chondroitin sulfate, glucosamine sulfate and their combination should be discouraged for the management of patients with symptomatic OA of the knee. This conclusion was based on the application of a “minimal clinically important difference” in pain reduction between active preparations and placebo (-9 mm on the 100 mm VAS). As reported in this study, the statistically significant difference between Structum® and placebo revealed by the meta-analysis approximated a difference of -6mm on the 100 mm VAS. This difference, based on the postulate by Wandel and coauthors (1), would be considered as meaningless while clinically irrelevant. There exists, however, a considerable problem with the definition of the so called “minimal clinically important difference” applied by Wandel and coauthors [1]. To justify the use of this “minimal clinically important difference” the authors refer to four publications on this subject [26 - 29] and in all of which the described “minimal clinically important differences” refer to differences in pain intensity before the start of treatment (baseline, non-treatment) and after a treatment period (endpoint), i.e. between treatment and non-treatment. Since it is known fact that the assessment of pain in OA is affected to a considerable degree by placebo effect [30, 31], the difference between treatment and non-treatment is not equivalent or comparable to an effect of active vs placebo treatment. Thus, the transfer of such a “minimal clinically important difference” from a given clinical situation into another one, not comparable clinical situation is scientifically not justifiable.
On the other hand, in an evaluation of clinically relevant states in patients with knee OA, patients considered their state satisfactory if their pain score was less than 32.3 mm (95% CI 30.1 to 34.7) on the 0-100 mm VAS [32]. In our meta-analysis, the mean VAS change in pain during activity between baseline and the end of study was -25.9 mm (95% CI -28.7 to -23.2) in the Structum® group. Based on baseline values of 54-61 mm VAS in the three studies evaluated, it is obvious that most of the patients receiving Structum® reached a pain score of less than 32.3 mm VAS, thus experienced a meaningful improvement, i.e. a clinically relevant reduction in pain at the end of treatment. Furthermore, a reduction in pain intensity represents one of the best investigated placebo effects within the context of evaluating clinical effectiveness [30, 33-35]. In a recently published meta-analysis [31] the placebo effect in randomized, controlled clinical trials in OA was evaluated involving 193 placebo treated groups consisting of a total of 16364 patients and 14 untreated control groups of a total of 1167 patients. For pain relief placebo treatment showed an effect size of 0.51 (95% CI, 0.46 - 0.55) whereas in untreated control groups the effect size approached zero (ES 0.03 (-0.13 - 0.18)). As a matter of fact, in „head-to-head“-trials of placebo vs non-treatment the effect size was even larger (ES 0.77 (0.65 - 0.89)). Placebo was also described to be effective for other endpoints such as perceived stiffness, self-reported function and physician’s global assessment.
CONCLUSION
In conclusion, the administration of chondroitin sulfate in the form of a specific preparation, i.e. Structum®, at an oral dose of 1 g/day is effective in reducing pain intensity during activity in patients suffering from symptomatic OA of the knee over treatment periods of 3-6 months. Furthermore, Structum® treatment improves functionality as judged by the Lequesne’s algo-functional index and increases the actual numbers of OMERACT-OARSI responders in a statistically significant fashion. Therefore, given the safety of chondroitin sulfate preparations in general in addition to a meaningful improvement with respect to pain, it seems justified to recommend the use of Structum® among the other symptom-slow acting drugs in OA for the control of symptoms in the management of symptomatic OA of the knee of Kellgren-Lawrence grades II and III.
CONTRIBUTION OF AUTHORS
Conception and design (M Cucherat, H Schneider).
Analysis and interpretation of the data (M Cucherat, H Schneider).
Drafting of the article (H Schneider).
Critical revision of the article for important intellectual content (H Schneider, M Cucherat, E Maheu).
Final approval of article (H Schneider, M Cucherat, E Maheu).
ACKNOWLEDGEMENTS
The work was supported by a grant from Laboratoires Pierre Fabre, Castres, France, to MC for extracting the data and performing the meta-analysis. Funds for writing this manuscript were provided by Robapharm AG, Allschwil, Switzerland (subsidiary of Laboratoires Pierre Fabre) to HS.
CONFLICT OF INTEREST
E. Maheu is the principal and lead investigator on a clinical trial that is presently getting started and is sponsored by Laboratoires Pierre Fabre, Castres, France, and is utilizing Structum®, in addition to hyaluronan, for knee OA.
M. Cucherat was supported by a grant from Laboratoires Pierre Fabre, Castres, France, for perfoming data extraction and meta-analyses.
H. Schneider received funds from Robapharm AG, Allschwil, Switzerland (subsidiary of Laboratoires Pierre Fabre) for writing the manuscript.


