All published articles of this journal are available on ScienceDirect.
Biosimilar Denosumab in Osteoporotic Patients Attending a Tertiary Care Hospital of Eastern India: A Real-world Comparative Retrospective Observational Study
Abstract
Background
Denosumab, a monoclonal antibody directed against RANK-Ligand, inhibits the formation, function, and survival of osteoclasts, leading to the inhibition of bone resorption, resulting in increased bone mass and prevention of fractures. Despite the availability of biosimilar denosumab in India for more than 5 years, Real-world Evidence (RWE) on the control of disease severity, tolerability, and safety of these biosimilars is lacking.
Objectives
This analysis is intended to compare the in-clinic effectiveness and safety of the Indian drug authority-approved denosumab biosimilar (Intas-Pharma) with the innovator denosumab.
Methods
Data of osteoporotic patients receiving either innovator denosumab or biosimilar denosumab (60mg SC every 6 months) for 2 years in routine clinical practice as per patient choice were evaluated. Effectiveness was measured based on the change in Lumbar spine (LS-TS) and Total Hip T scores (HIP-TS). Student t-test was carried out to evaluate intragroup change in T-scores at both sites and compare inter-group T-scores at baseline, as well as after 2 years of denosumab treatment at both sites. Adverse events reported in both groups were noted.
Results
A total of 49 osteoporotic patients receiving denosumab (n=28 biosimilar denosumab, n=21 innovator denosumab) were evaluated. All patients received concomitant vitamin D and calcium throughout the study duration.
The mean LS-TS values for the innovator and biosimilar groups at baseline were -3.85±0.20 and -3.82±0.21, respectively, while the mean HIP-TS values for the innovator and biosimilar group at baseline were -3.60±0.22 and -3.57±0.21, respectively. Both groups were comparable at baseline, and there was no significant difference between the two groups in terms of T scores at both sites. After 2 years of denosumab treatment, mean LS-TS values for the innovator and biosimilar groups reached -2.82±0.26 and -2.74±0.25, respectively, while the mean HIP-TS values for the innovator and biosimilar group were recorded to be -2.41±0.27 and -2.40±0.24, respectively. When post-treatment T scores were compared, there was no significant difference between the two groups at any of the sites.
When an intra-group change in T scores from baseline over 2 years was evaluated, both innovator and biosimilar denosumab groups were observed to have significant improvement (p<0.0001) in mean LS-TS as well as HIP-TS values. No severe adverse effects were noted in any group, and the safety profile of both groups was comparable.
Conclusion
Biosimilar denosumab was found to be as effective as innovator denosumab in increasing bone mineral density at the lumbar spine as well as total hip among osteoporotic patients attending Indian rheumatology clinics.
1. INTRODUCTION
Osteoporosis is a skeletal disorder characterized by low bone mineral density and degradation of bone structure, which leads to decreased bone strength, resulting in increased susceptibility to fractures [1]. The prevalence of osteoporosis is 8 to 62% among Indian females, as reported by various studies [2]. In postmenopausal women, this prevalence is much higher because of low levels of estrogen, which results in increased loss of bone mass [3]. Though it is prevalent in both genders, women are more prone to develop osteoporosis and incur fragility fractures. In the Eastern region of India, the prevalence of osteoporosis is around 18.4%, with a similar male-to-female ratio [4]. The outcomes of osteoporosis are mainly fragility fracture and chronic pain [3].
Management strategies for osteoporosis include both non-pharmacological and pharmacological methods. Non-pharmacological management options include an adequate amount of vitamin D and calcium intake, decreased consumption of alcohol/caffeine, weight-bearing exercise, quitting smoking, and utilizing fall avoidance techniques [5]. The aim of pharmacological treatment is to decrease the chances of fracture by either increasing bone formation by anabolic agents like Teriparatide or reducing the bone resorption process with the help of anti-resorptive agents like bisphosphonates, denosumab (RANK ligand inhibitor), estrogen, and Selective Estrogen Receptor Modulators (SERMs) [6].
In postmenopausal osteoporotic women at high risk of fracture, most of the guidelines suggest bisphosphonates as an initial treatment based on their safety, efficacy, benefits, affordability, and long-lasting effects after discontinuation [7]. Newer agents like denosumab have been recommended as an initial treatment option in postmenopausal osteoporotic patients who are at a strong risk for osteoporotic fractures [8], especially among patients with deranged kidney function and intolerance to bisphosphonates.
Denosumab is a fully human monoclonal antibody that acts against the ligand of receptor activator of nuclear factor kappa-B (RANK) receptors [9]. By virtue of its specificity for RANK-L, it reduces the activation of RANK, thereby leading to inhibition of osteoclast recruitment, formation, function, and survival, which ultimately manifests as a slowing down of bone resorption [9].
Sufficient data is available to endorse the efficacy and safety of denosumab in postmenopausal osteoporosis. Several studies have shown its efficacy to be superior to bisphosphonates in terms of an increase in Bone Mineral Density (BMD) [10]. However, the main barrier against the more widespread use of denosumab in India is the high cost of the innovator molecule. To overcome this barrier, pharmaceutical companies in India have been involved in the development of denosumab biosimilars for a long time.
Intas Pharmaceuticals developed a denosumab biosimilar, where biosimilarity with the reference product was established through physicochemical and biological characterization and clinical and non-clinical studies [11].
Intas received approval for marketing authorization of biosimilar Denosumab from the Drug Controller General of India in 2017 [12]. In a phase III study involving biosimilar denosumab, the BMD at the lumbar spine improved by 7.22% with denosumab-biosimilar and by 7.62% with denosumab-innovator over the period of 12 months. There was no significant difference between the two values. The improvements in hip BMD and bone-specific alkaline phosphatase levels were also similar among both groups [11].
Despite the availability of biosimilar denosumab for more than 5 years in India, real-world evidence on the control of disease severity, tolerability, and safety of these biosimilars is lacking. The objective of this study was to compare the in-clinic effectiveness and safety of DCGI-approved denosumab biosimilar developed by Intas Pharmaceuticals Ltd. with innovator denosumab.
2. METHODS
A retrospective, observational study was conducted on adult Indian osteoporotic patients attending the out-patient department of a tertiary care hospital in eastern India who were receiving either innovator denosumab or biosimilar denosumab (60mg S/C every 6 months) for the treatment of osteoporosis, as per patient’s choice. Data regarding T scores at LS spine and total hip at baseline and after 2 years of denosumab therapy (either innovator denosumab or the biosimilar molecule) were retro- spectively collected. Patients in both groups received 800 IU of vitamin D and 1000 mg of calcium throughout the study duration. A paired student t-test was carried out to evaluate intragroup change in T-scores at both sites (LS Spine and Total Hip) for both innovator denosumab and biosimilar denosumab groups. An unpaired t-test was conducted to compare inter-group T-scores at baseline and after 2 years of treatment for both groups to detect any difference between innovator denosumab and biosimilar denosumab in T score improvement. Data regarding adverse effects seen with therapy in both groups were also collected. The study was conducted after obtaining clearance from the Institutional Ethical Committee of Apollo Multispecialty Hospitals Limited. The reference number for this study is IEC/BR/2024/01/02, and the research work was carried out in accordance with the Helsinki Declaration. A waiver of informed consent was granted for this retrospective study, provided no identifiers were recorded for research purposes.
3. RESULTS
A total of 49 osteoporotic patients receiving denosumab 60 mg every 6 months (n=28 biosimilar denosumab, n=21 innovator denosumab) were evaluated. All patients concomitantly received 800 IU of vitamin D and 1000 mg of calcium throughout the study duration. The baseline characteristics and demographic profiles of the patients were similar.
3.1. Improvement in Bone Health by Biosimilar
The mean lumbar spine T-score at baseline for denosumab biosimilar was -3.82±0.21. After 2 years of treatment, the mean LS-TS reached -2.74±0.25. The total hip T score at baseline was -3.57±0.21, which improved to -2.40±0.24. The improvement in T scores at both sites was statistically significant.
3.2. Comparative Effectiveness and Safety between Innovator and Biosimilar
The mean LS-TS values for the innovator and biosimilar groups at baseline were -3.85±0.20 and -3.82±0.21, respectively, while the mean HIP-TS values for the innovator and biosimilar group at baseline were -3.60±0.22 and -3.57±0.21, respectively. Both groups were comparable at baseline, and there was no significant difference between the two groups in terms of T scores at both sites. After 2 years of denosumab treatment, mean LS-TS values for the innovator and biosimilar groups reached 2.82±0.26 and -2.74±0.25, respectively, while the mean HIP-TS values for the innovator and biosimilar group were recorded to be -2.41±0.27 and -2.40±0.24, respectively. When post-treatment T scores were compared, there was no significant difference between the two groups at any of the sites (Table 1).
When an intra-group change in T scores from baseline over 2 years was evaluated, both innovator and biosimilar denosumab groups were observed to have significant improvement (p<0.0001) in mean LS-TS as well as HIP-TS values (Fig. 1).
| Site | Baseline T Score |
P-value (T scores for both groups at baseline) |
T score at 2 years |
P-value (T scores for both groups at 2 years) |
||
|---|---|---|---|---|---|---|
| Innovator group | Biosimilar group | Innovator group | Biosimilar group | |||
| Lumbar Spine | -3.85±0.20 | -3.82±0.21 | 0.62 | 2.82±0.26 | -2.74±0.25 | 0.26 |
| Total Hip | -3.60±0.22 | -3.57±0.21 | 0.55 | -2.41±0.27 | -2.40±0.24 | 0.81 |
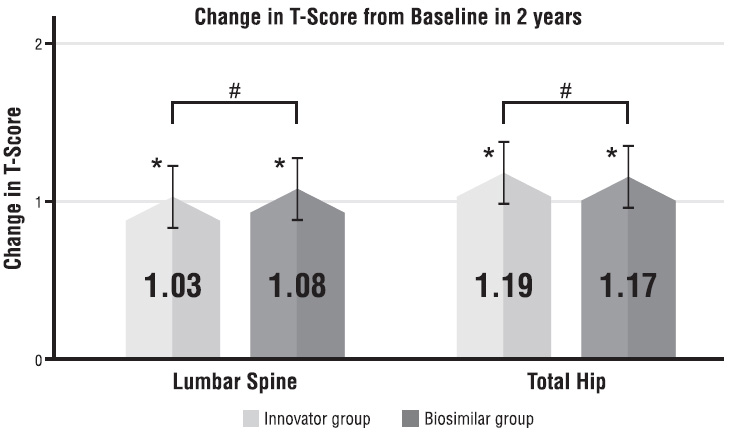
Change in T score from baseline in 2 years among innovator and biosimilar denosumab group at the lumbar spine and total hip.
*p<0.0001 comparing 2-year data from baseline; #p>0.05 comparing inter group change in T scores.
Common adverse reactions noted in both groups were back pain, pain in extremities, musculoskeletal pain, hypercholesterolemia, and cystitis. Moreover, the safety profiles of both groups were comparable.
4. DISCUSSION
Denosumab is a potent antiresorptive medication used for the management of osteoporosis, with clinical trial data of 10 years demonstrating its safety and efficacy [13]. It is a monoclonal antibody, which is the first and only RANKL inhibitor that has received approval for the treatment of postmenopausal osteoporosis. Initial marketing authorization was received as early as 2010 in various countries, such as the US, the European Union, and many others [11]. The efficacy of denosumab in improving BMD and reducing fracture risk has been adequately demonstrated in a number of studies.
In the DATA study, which was carried out on postmenopausal osteoporotic women, it was found that lumbar spine BMD, femoral neck BMD, and total hip BMD were significantly improved with innovator denosumab after 12 months of treatment [14]. In the FREEDOM Study, it was found that denosumab had substantially decreased risk of vertebral, non-vertebral, and hip fractures [13].
The main drawback of denosumab therapy, especially in an emerging economy like India, is the price factor. To overcome the limitations posed by the high price of innovator denosumab, several denosumab biosimilars have been developed.
Studies involving these biosimilar molecules have also been documented. A Chinese study showed a significant improvement in lumbar spine BMD from baseline to after 1 year of usage of biosimilar denosumab LY06006 [15].
Another study demonstrated the non-inferiority of biosimilar denosumab (Arylia) as compared to the reference molecule and showed a comparable safety profile at 18 months [16]. Similarly, biosimilar denosumab developed by Intas Pharmaceuticals was found to have efficacy comparable to that of the innovator molecule [11].
Denosumab biosimilar has been available in India for more than 5 years, but sufficient real-world evidence related to its effectiveness and safety is still not available. So, this retrospective observational study was carried out to understand its effect in real-world scenarios.
The current retrospective observational study compared the improvement in T scores at the lumbar spine and total hip with biosimilar and innovator denosumab. There was no difference in the T score between the innovator and biosimilar group at baseline. However, a significant and similar improvement in mean T score was found in both innovator and biosimilar groups at both sites in 2 years.
Common adverse reactions noted in both groups were back pain, pain in extremities, musculoskeletal pain, hypercholesterolemia, and cystitis. None of the reported adverse events were serious, and all AEs were resolved in due course. Both innovator and biosimilar denosumab had similar safety profiles. The adverse events noted in the current study are in line with the known safety profile of denosumab as per the previously conducted study on innovator denosumab.
5. LIMITATIONS
While our investigation revealed encouraging findings concerning the real-world efficacy and safety of biosimilar denosumab in treating osteoporosis, it is crucial to acknowledge certain limitations that merit consideration. The small patient pool in this study was a limitation besides a relatively short duration of 2 years. Additionally, the retrospective nature of the study also increases some of the research biases.
CONCLUSION
This study leads to the conclusion that Indian denosumab biosimilar has similar safety and effectiveness compared to innovator denosumab when used in real-world scenarios. Comparable real-world effectiveness and safety of biosimilar denosumab as a cheaper alternative will help increase its affordability and widen its usage.
AUTHORS’ CONTRIBUTION
S.B.: Study conception and design; A.G.D.: Data collection; S.C.: Writing the paper; P.S., S.S., G.S.: Writing the reviewing and editing; A.D.: Investigation; R.S.: Validation. All authors reviewed the results and approved the final version of the manuscript.
LIST OF ABBREVIATIONS
| RWE | = Real-World Evidence |
| HIP-TS | = Hip T scores |
| SERMs | = Selective Estrogen Receptor Modulators |
| BMD | = Bone Mineral Density |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was conducted after obtaining clearance from the Institutional Ethical Committee of Apollo Multispecialty Hospitals Limited Kolkata, India. The reference number for this study is IEC/BR/2024/01/02.
HUMAN AND ANIMAL RIGHTS
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
A waiver of informed consent was granted for this retrospective study, provided that no identifiers were recorded for research purposes.
AVAILABILITY OF DATA AND MATERIAL
All data generated or analyzed during this study are included in this published article.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Amit B Jain (Intas Pharmaceuticals Limited, Ahmedabad, India) for providing scientific writing assistance and critically reviewing the manuscript at various stages.


