All published articles of this journal are available on ScienceDirect.
Multiple Hepatic Micro-Hypodensities as a Presenting Sign in Systemic Lupus Erythematosus- A Case Report
Abstract
Systemic Lupus Erythematosus (SLE) is a chronic multisystemic inflammatory disorder that can present with a wide array of signs and symptoms. Hepatic involvement is commonly limited to a subclinical biochemical transaminitis while clinically significant liver disease is rare. A case of a 22-year-old female who presented with abdominal pain, fevers, arthralgia, and several hepatic hypodense lesions with normal liver function tests is reported in this study. She failed to improve with antibiotics and infectious workup was largely unrevealing. She was found to have a positive ANA, high titers of anti-double-stranded DNA antibody, and was ultimately diagnosed with new-onset SLE with hepatic aseptic micro-abscesses. Her symptoms were self-limiting, and she was later started on a low-dose prednisone taper and hydroxychloroquine. This case demonstrates that hepatic involvement, despite normal liver function tests, should be considered in SLE patients presenting with abdominal pain.
1. INTRODUCTION
Systemic Lupus Erythematosus (SLE) is a complex chronic inflammatory disorder often with multi-organ involvement. The underlying etiology is unknown, and pathophysiology involves the production of several pathological auto-antibodies. Any organ system from musculoskeletal, renal, cutaneous, cardiovascular, hematological, and CNS has known to be affected in SLE. Gastrointestinal (GI) involvement has been described in various forms, from enteritis and protein-losing enteropathy to small bowel obstruction [1-3]. The most common hepatic injury in SLE is a subclinical transaminitis in either hepatocellular or cholestatic patterns [2-5]. Here, a case of a young woman with normal liver function tests presenting with multiple hepatic lesions directly related to an initial presentation of SLE, is reported.
2. CASE PRESENTATION
A 22-year-old female presented to the emergency room with abdominal pain and headache. Her symptoms began ten days ago with sharp, non-radiating right upper quadrant abdominal pain that progressively worsened to 7/10 in severity. There was no association with food intake, loss of appetite, diarrhea, nausea, or vomiting. She denied any change in bowel
habits or characteristics. Three days after the onset of pain, she developed several symptoms a sore throat with multiple painful oral ulcers, diffuse arthralgia, non-productive cough, intermittent fevers up to 102° F, and a 15lb weight loss in the last two months. She denied any Raynaud’s phenomenon, joint swelling, or skin changes. She reported a history of occasional painful vaginal ulcers in the past and noticed them a few days prior that seemed to have resolved. She was previously tested for sexually transmitted diseases and was found to be negative for HSV, gonorrhea/chlamydia, and HIV.
Four days before admission, she presented to local urgent care and was diagnosed with an acute viral syndrome. She was started on amoxicillin/clavulanic acid for a possible developing bacterial respiratory infection. Other than an improvement in her oral ulcers, she denied any change in her symptoms and represented to the emergency room for ongoing abdominal pain and a new frontal headache. The headache was constant and minimally responsive to acetaminophen. She had no associated vision/hearing changes, vertigo, auras, and this was not like her baseline migraines.
Her medical history was notable for migraines, as mentioned above, and she had a strong family history of autoimmunity. Her father had Grave’s disease, and mother and maternal aunt had SLE. She was sexually active and used barrier contraception with a monogamous partner. She denied any recent travel, new or undercooked foods, sick contacts, significant smoking, alcohol use, or illicit drug use.
Her initial vital signs were: blood pressure of 115/76 mmHg, heart rate of 75, temperature of 36.8 °C, respiratory rate of 18 breaths/min, and oxygen saturation of 97% on room air. She appeared non-toxic, well-nourished, and well-developed. No oral or genital ulcers were observed. The cardiac and pulmonary exam was unremarkable. An abdominal exam was notable for normoactive bowel sounds and moderate-severe right upper quadrant tenderness without any rebound tenderness, distention, palpable masses, or hepatosplenomegaly. There were no rashes, jaundice, or skin pallor. There was no palpable lymphadenopathy. The musculoskeletal exam was also unremarkable with an intact range of motion and no extremity edema, synovitis, or weakness. There was no palpable nuchal rigidity, and Brudzinski's and Kernig signs were negative. The remainder of the exam was also unremarkable.
The initial differential focused on an infectious etiology. Pertinent results are noted in Tables 1-3. No table labs were: serum white blood cell count 12.5 K/mcL, hemoglobin 12.0 g/dL, platelets 262 K/mcL, creatinine of 0.7 mg/dL, aspartate aminotransaminase 25 U/L, alanine aminotransaminase 12 U/L, total bilirubin 0.4 mg/dL, partial thromboplastin time 12.6 seconds, international normalized ratio 1.1, albumin 4.1 g/dL and total protein of 7.4 g/dL. Microscopic urinalysis was notable for no proteins/casts, 3-5 WBC/HPF, small leukocyte esterase, negative nitrites, and negative for urine hCG. Since she also complained of a new headache, we performed a lumbar puncture. The results are as follows: CSF protein 13 mg/dL, glucose 62 mg/dL, cell count with 1 nucleated cell and 93 red blood cells/mcL, and a negative gram stain. MRI of her brain and cervical spine did not reveal any concerning findings.
| - | Result (ref range) | - | Result (ref range) |
|---|---|---|---|
| WBC | 12.5 K/mcL (3.4-11 K/mcL) | Albumin | 4.1g/dL (3.5-5 g/dL) |
| Hgb | 12.0 g/dL (11.9-15.3 g/dL) | Total Protein | 7.4 g/dL (6.3-8.2 g/dL) |
| MCV | 86 fL (81-100 fL) | ALP | 49 U/L (38-126 U/L) |
| Plt | 262 K/mcL (150-425 K/mcL) | AST | 25 U/L (14-36 U/L) |
| Abs Lymphocyte | 1.25 K/mcL (0.9-3.10 L/mcL) | ALT | 12 U/L (<35 U/L) |
| Abs Neutrophil | 10.32 K/mcL (1.5-7.4K/mcL) | Total bilirubin | 0.4 mg/dL (0.2-1.3mg/dL) |
| Na+ | 140 mmol/L (137-145 mmol/L) | Urine Pregnancy | Negative |
| K+ | 4.0 mmol/L (3.5-5.1 mmol/L) | Urine WBC | 3-5/HPF |
| BUN | 7 mg/dL (7-17 mg/dL) | Urine leukocyte esterase | Small |
| Cr | 0.7 mg/dL (0.5-1.0 mg/dL) | Urine Nitrite | Negative |
| Calcium | 8.9 (8.4-10.2 mg/dL) | Urine Protein/Casts | Negative |
| LDH | 334 U/L (313-618 U/L) | PT | 11.8 sec (9.4-11.6 sec) |
| CSF glucose | 62 mg/dL (40-70 mg/dL) | INR | 1.1 (0.9-1.1) |
| CSF cell count | 1 nucleated cell, 93 RBC (< 11/mcL) | CSF gram stain | Negative |
| CSF Protein | 13 mg/dL (12-60 mg/dL) |
| - | Result | - | Result |
|---|---|---|---|
| Hepatitis B surface Ag | Negative | Vaginitis PCR* | Negative |
| Hepatitis B core IgM | Negative | GC/Chlamydia | Negative |
| Hepatitis C antibody | Negative | Influenza A/B PCR | Negative |
| Hepatitis A IgM | Negative | QuantiFERON TB | Negative |
| HIV p24 Ag/IgG Ab | Negative | MonoSpot | Negative |
| EBV PCR | Negative | RPR | Negative |
| CMV PCR | Negative | Coccidioides IgG, IgM | Negative |
| HSV PCR | Negative | Cryptococcal Ag | Negative |
| CSF culture | Negative | Histoplasma Ag | Negative |
| Blood cultures | Negative | 1,3 Beta-D glucan/galactomannan | Negative |
| - | Result (ref range) | - | Result (ref range) |
|---|---|---|---|
| Sedimentation Rate | 59 mm/hr (0-29mm/hr) | C-reactive protein | 88.9 mg/L (<10mg/L) |
| ANA | Positive, homogenous 1:40 (< 1:40) | Anti-double stranded DNA | Positive, 118 IU (0-24 IU) |
| ANCA, PR3/MPO | Negative | Anti-Smith/RNP | Negative |
| C3 | 72 mg/dL (90-180 mg/dL) | C4 | 15 mg/dL (10-40mg/dL) |
| SSA/SSB | 0.5 U/mL (<7.0 U/mL) | DRVVT Ratio | 3.44 (<1.12) |
| Anti-cardiolipin, IgG | < 10 GPL (0-14 GPL) | CPK | 25 U/L (0-175 U/L) |
| Rheumatoid Factor | < 10IU/mL (0-13 IU/mL) | Ferritin | 104 ng/mL (13-150ng/mL) |
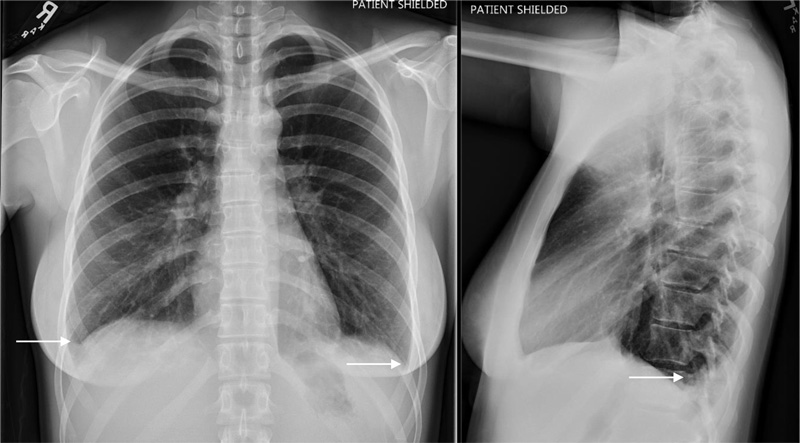
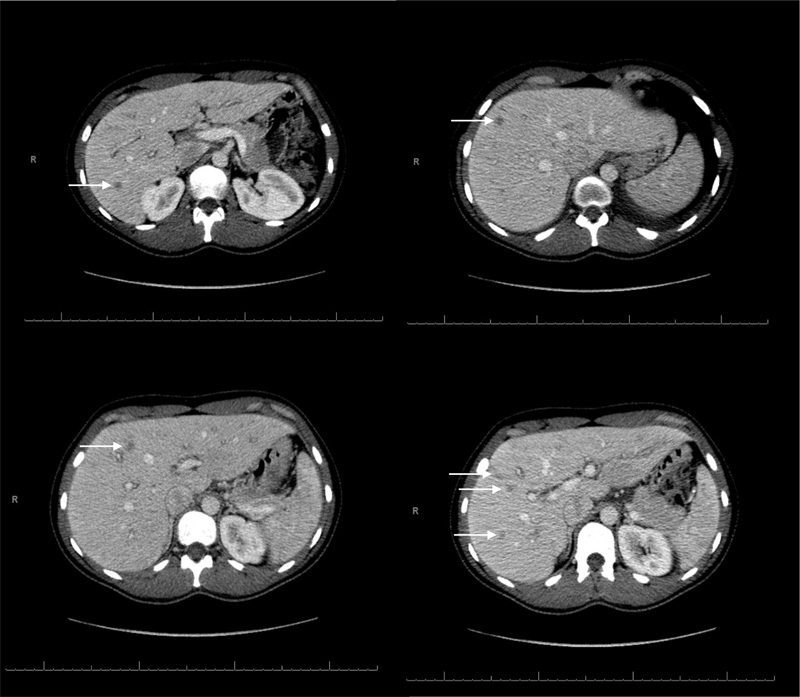
Chest x-ray was notable for new mild bilateral pleural effusions (Fig. 1). Right upper quadrant ultrasound with doppler did not reveal any concerning lesions, and portal vasculature was patent. Subsequent computerized tomography of her abdomen and pelvis without contrast revealed several hepatic lesions concerning micro-abscesses (Fig. 2).
Pertinent results of the infectious workup were: hepatitis A IgM negative, hepatitis B surface antigen and core antibody negative, hepatitis C antibody negative, HIV p24 antigen/IgM/IgG negative, QuantiFERON gold negative, histoplasma antigen negative. The remainder of the infectious workup is listed in Table 2, but it was overall unrevealing. Based on the negative infectious workup, the focus was subsequently shifted to a rheumatological workup detailed in Table 3: estimated sedimentation rate 59 mm/hr, c-reactive protein 88.9, anti-neutrophil antibody 1:40, anti-double stranded DNA 118 IU, anti-nuclear cytoplasm antibody negative-PR3/MPO negative, anti-Sjogren's syndrome A/B negative, anti-smith antibody negative, anti-cardiolipin IgG < 10, dilute Russell viper venom time screen ratio (DRVVT) 3.44, C3 72 mg/dL, C4 15 mg/dL, rheumatoid factor < 10, and ferritin 104 ng/mL. Unfortunately, no biopsy was done due to the size and characteristics of the lesions.
She was started on broad-spectrum antibiotics with intravenous vancomycin, cefepime, and metronidazole for possible pyogenic vs. amoebic hepatic abscesses. However, she did not improve following three days of IV antibiotics, and her abdominal pain and arthralgia persisted. Based on her strong family history, clinical symptoms, positive anti-dsDNA, positive ANA, low C3, and elevated DRVVT, she was ultimately diagnosed with new-onset SLE. Parenteral corticosteroids were not initially started due to ongoing concern for an underlying infectious process. Her arthralgia, abdominal pain, and fevers started to improve spontaneously with naproxen prior to initiation of parenteral corticosteroids, hence they were not administered, and she was monitored closely. She was discharged on hospital day eight and started on hydroxychloroquine and low-dose prednisone taper as an outpatient with significant clinical improvement.
3. DISCUSSION
Gastrointestinal involvement has been well described and nearly 40% of all individuals with SLE are reported to have some form of GI involvement in their lifetime [3]. Hepatic involvement is commonly seen as a non-specific transaminitis with either cholestatic or hepatocellular pattern, but clinically significant hepatic dysfunction from SLE is thought to be uncommon [4, 5]. In one review of 40 SLE patients with clinically significant hepatic dysfunction, the underlying etiologies were found to be due to: primary biliary cholangitis (n=3), drug-induced (n=4), viral hepatitis (n=8), NAFLD (n=8), autoimmune hepatitis (n=8) and miscellaneous (n=11)- liver involvement from infection [2], cryptogenic cirrhosis [2], lymphoma [1], and indeterminate [6]. Following a median follow-up of 44 months, 5-year serious liver disease-free survival was 93% [5]. Runyon et al. reported that out of 238 SLE patients, 60% of patients were found to have at least one liver function test abnormality, but only 20% showed any existence of a clinical liver disease. The diagnosis of liver disease in that cohort occurred either up to four years before or five years after the diagnosis of SLE [4]. In another retrospective review of 100 SLE patients with transaminitis, only 23% showed an elevation in liver function test thought to be due to SLE hepatic involvement with no other explainable cause for the liver disease [6]. These reports highlight that liver function abnormalities in SLE patients often may have another underlying etiology to explain their abnormality, and true hepatic involvement from SLE is less common. Moreover, frank hepatic lesions in SLE are a rare subset in true hepatic involvement. It is suspected the hepatic lesions in this case were related to a new SLE diagnosis since no other discernable etiology was found. Alanazi et al. reported a similar case in a young woman with SLE who developed right upper quadrant pain, elevated liver function tests, and later was found to have hepatic vasculitis mimicking as liver abscesses. Biopsy of that hepatic lesion showed generalized mild chronic inflammation [7]. Miyake et al. also reported a patient with distinct liver densities and elevated liver function tests that were concerning for lupus-related vasculitis. Biopsy showed inflammatory cell infiltration and subtle necrosis [8]. Both of those cases were treated with high dose corticosteroids with improvement in symptoms and lesions. This case reflected either an underlying vasculitic process or aseptic micro-abscesses directly related to underlying SLE. Unfortunately, no biopsy was done to help aid the diagnostic process. Aseptic liver abscesses have been described in inflammatory bowel disease, particularly Crohn’s, but are rare in systemic inflammatory conditions [9]. Maeshima et al. reported a case of aseptic abscesses in a patient with Behcet’s disease that improved with corticosteroid therapy [10, 11]. The diagnosis of Behçet’s was considered based on her reported painful oral and vaginal lesions but similar lesions in SLE have also been reported [12]. Ultimately, she satisfied the 2019 ACR/EULAR criteria for SLE with positive ANA, arthralgias, mucosal ulcers, pleural effusion, elevated anti-dsDNA, and elevated DRVVT [13, 14]. However, it was noted that her ANA titer is below the recognized titer of 1:80 noted in the most recent ACR/EULAR criteria. Her diagnosis was corroborated by high titers of anti-dsDNA antibody that has a reported specificity of up to 97.4% and a positive LR > 10 for SLE and her overall clinical picture in this setting was felt to be sufficient to meet the diagnosis of SLE [11].
This case interestingly did not present with a transaminitis. The studies discussed above involved some form of liver function test abnormality consistent with common hepatic findings. This case may have been in its early stages and had not yet reached a point of precipitating a transaminitis, but her serological studies and clinical presentation was consistent with a flare and not a subclinical state suggesting that hepatic involvement may not reflect the underlying stage of SLE.
CONCLUSION
SLE can be difficult to diagnose due to the variability in presentation, organ system involvement, and seropositivity. Symptoms can be non-specific and serological workup can often only be suggestive. Hepatic involvement should be considered if transaminitis is present and not explained by other more common etiologies such as infectious, ischemic, or local inflammatory. Autoimmune hepatitis (AIH) is often a consideration and was on differential. AIH is thought to have distinct pathophysiology from hepatic involvement in SLE but can present similarly with transaminitis, arthralgia, and constitutional symptoms. Apart from ANA, several different auto-antibodies-notably anti-smooth muscle, anti-actin, anti-SLA/LP, and anti-mitochondrial can be involved in AIH and usually does not involve anti-phospholipid antibodies. Moreover, there are no characteristic imaging findings in AIH, unlike with SLE, as seen in this case. Imaging findings are often unremarkable and do not aid in the diagnosis of AIH. AIH histopathological studies often show abundant plasma and lymphocytic infiltration with necrosis compared to SLE involvement, which tends to have milder inflammation with a paucity of lymphocytes. The prognosis without treatment is poor in AIH and can lead to cirrhosis compared to hepatic involvement in SLE, which can be benign [15, 16].
Overall, hepatic involvement varies in degree and character in SLE and should be considered in patients with abdominal symptoms, even in the setting of normal liver function tests. The severity of hepatic involvement can vary and may not reflect the severity of underlying SLE.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
HUMAN AND ANIMAL RIGHTS
Not applicable.
CONSENT FOR PUBLICATION
Informed consent was taken from all the participants when they were enrolled.
STANDARDS OF REPORTING
CARE guidelines have been followed in this study.
FUNDING
None.
CONFLICT OF INTEREST
The authors have no relevant conflicts of interest or financial supports to disclose. To protect patient privacy, identifying information including names, initials, or hospital rooms are not described in manuscript and figures.
ACKNOWLEDGEMENTS
Declared none.


