RESEARCH ARTICLE
Predictor of the Simplified Disease Activity Index 50 (SDAI 50) at Month 3 of bDMARD Treatment in Patients with Long-Established Rheumatoid Arthritis
Yusuke Miwa1, *, Mayu Saito1, Hidekazu Furuya1, Ryo Yanai2, Tsuyoshi Kasama2
Article Information
Identifiers and Pagination:
Year: 2017Volume: 11
First Page: 106
Last Page: 112
Publisher ID: TORJ-11-106
DOI: 10.2174/1874312901711010106
Article History:
Received Date: 15/05/2017Revision Received Date: 21/06/2017
Acceptance Date: 12/09/2017
Electronic publication date: 30/09/2017
Collection year: 2017

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: (https://creativecommons.org/licenses/by/4.0/legalcode). This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objectives:
The Simplified Disease Activity Index (SDAI) 50 has good agreement with European League Against Rheumatism (EULAR) response measures for early Rheumatoid Arthritis (RA). There have been reports on early RA, but not on long-established RA. In this study, we analysed the relationships between various baseline factors and SDAI 50 after three months of treatment with biological disease-modifying antirheumatic drugs (bDMARDs) to determine the prognostic factors for long-established RA.
Methods:
Subjects were 260 RA patients who had been treated with bDMARDs for 3 months. The following characteristics were investigated: Patient backgrounds, the erythrocyte sedimentation rate (ESR), C-reactive protein and serum matrix metalloproteinase-3 levels, SDAI scores, and health assessment questionnaire disability index and short form-36 scores. As a primary outcome index, the SDAI response was defined as a 50% reduction in the SDAI score between baseline and 3 months (SDAI 50).
Results:
Baseline values of disease duration (odds ratio: 0.942, 95% CI: 0.902-0.984), smoking history (odds ratio: 2.272, 1.064-4.850), 28-tender joint count (odds ratio: 0.899, 0.827-0.977), evaluator's global assessment (odds ratio: 1.029, 1.012-1.047) and ESR (odds ratio: 1.015, 1.001-1.030) were determined to be significant factors based on logistic regression analysis.
Conclusion:
Our study demonstrated that RA patients with shorter disease duration, no smoking, and higher RA disease activity are more likely to achieve SDAI 50 through bDMARD treatment.
1. INTRODUCTION
Recommendations for the treatment of rheumatoid arthritis (RA) are well established [1]. The use of methotrexate (MTX) as an anchor agent, in combination with biological disease-modifying antirheumatic drugs (bDMARDs), has contributed to an increasing number of patients who achieve clinical remission. As a result of this increase in the rate of clinical remission, the number of patients achieving structural and functional remission has also increased [2]. Complete remission in a patient is defined as the achievement of clinical, structural, and functional remission [3].
Predictors of clinical remission in RA have been reported [4, 5]. Current smoking, female sex, longer symptom duration and younger age predict a worse response to MTX in patients with new onset RA [6]. Aiming for Simplified Disease Activity Index (SDAI) [7]remission at month 6 is an appropriate strategy to obtain good functional and structural outcomes at month 12 [8]. Female sex, greater pain, and a lack of initial DMARD therapy reduced the probability of sustained remission [9].
The SDAI 50 shows good agreement compared with the European League Against Rheumatism (EULAR) response measures with early RA. The SDAI 50 response at 3 months appears to be a more significant predictor of outcomes at 6 months than the EULAR response [10]. For example, if the SDAI score improves from 24 to 12, SDAI 12 and 24 reflects moderate disease activity. However, a clinical improvement in the SDAI score from 24 to 12 is beneficial to patients. Compared to EULAR improvement criteria using a disease activity score (DAS) of 28, the SDAI 50 is an index that is easy to calculate and to use in everyday clinical practice. To make the point that SDAI 50 is more sensitive, also show comparable DAS 28 scores where response is not attained. Although multiple studies on clinical remission have been reported, there have been few reports on SDAI 50 response. There have been reports on early RA, but none on long-established RA.
In this study, we analysed the relationships between various baseline factors and SDAI 50 response after three months of bDMARD treatment to determine the prognostic factors in long-established RA.
2. MATERIALS AND METHODS
A retrospective multi-centre study was conducted in the Division of Rheumatology Department of Medicine Showa University Hospital, Showa University Koto-Toyosu Hospital and Showa University Northern Yokohama Hospital. RA patients who initiated bDMARD treatment from 1 January 2007 to 31 March 2016 were examined. Among the 260 patients undergoing bDMARD treatment, 241 were deemed eligible to participate as subjects in this study. Because of missing data, end so on, 19 patients were excluded (Fig. 1). The bDMARDs used in the study included infliximab for 95 patients, etanercept for 60 patients, adalimumab for 54 patients, golimumab for 28 patients, and certolizumab-pegol for 23 patients. The selection of bDMARDs was left to the primary physician.
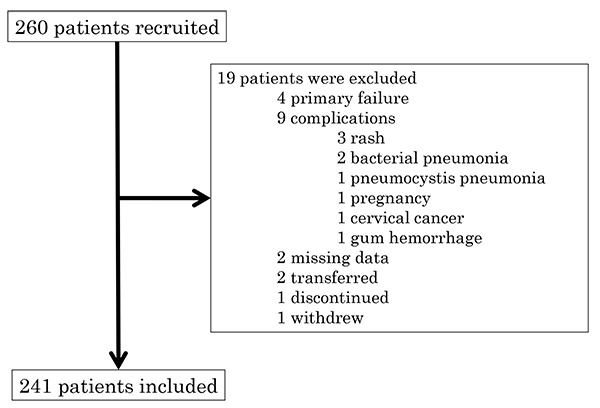 |
Fig. (1). Flow chart of the study. |
The following variables were evaluated at baseline (before the initiation of bDMARD treatment): Age, sex, disease duration, smoking history, body mass index (BMI), experience of bDMARD usage (bio-naïve or bio-switch), steroid dosage, MTX dosage and the combined use of conventional synthetic DMARDs (csDMARDs).
The following variables were evaluated at baseline and after 3 months: 28-swollen joint count (SJC), 28-tender joint count (TJC), patient global assessment (PTGA), evaluator global assessment (PHGA), C-reactive protein (CRP) levels, the erythrocyte sedimentation rate (ESR), and matrix metalloproteinase-3 (MMP-3) levels. Disease activity was evaluated using the SDAI. Activities of daily living (ADL) were evaluated using the health assessment questionnaire disability index (HAQ-DI) [11], and nonspecific health-related quality of life (QOL) was evaluated using the Short Form 36 (SF-36) [12]. The use of adrenocortical steroids (steroids) and non-steroidal anti-inflammatory drugs (NSAIDs) and their dosages before the initiation of bDMARD treatment as well as patient age and disease duration were not exclusion criteria.
As a primary outcome index, SDAI response was defined as a 50% reduction in the SDAI score between the baseline and 3 months (SDAI 50) [10]. To determine the relationship between baseline factors and SDAI 50 outcomes, the baseline values of each item were analysed based on whether SDAI 50 was achieved. The study exclusion criteria included the following: Tocilizumab abatacept and the use of bio-similar compounds; discontinuation of bDMARDs due to primary response failure or adverse effects; additional oral treatment using csDMARD agents or steroids; complications, such as infection; and the likelihood of not continuing the study because of situations such as hospital transfer, patient withdrawal from the study, or other patient circumstances that the primary physician deemed inappropriate for the study. For excluded patients, Last Observation Carried Forward (LOCF) analysis was not performed because of the severe missing data. Although data is available at the time of the start of treatment, the data after the start of treatment is not.
All of the statistical analyses were performed using univariate analyses and multivariate analyses with JMP12 software (SAS Institute Inc., Cary, NC, USA). We obtained written informed consent from all patients who enrolled in the study. The study received approval from the Bio-Ethics Committee of the Department of Medicine, Showa University School of Medicine (No. 1435).
3. RESULTS
Two-hundred-sixty patients were included in the study, as shown in Fig. (1). Primary failure patients were discontinued within 3 months of the initiation of the treatment and were thus excluded from analysis. In patients with complications, transfers, or discontinuation, the data at the time of discontinuation were missing.
In terms of background parameters for study subjects, 139 patients achieved SDAI 50 (Group A) and 102 did not (Group B) (Table 1). Based on univariate analyses, Group A had a significantly lower smoking history (p=0.038), higher ESR (p=0.008), SJC (p=0.038), PHGA (p=0.020) and SDAI (p=0.006) than Group B. No other factors showed significant differences between Groups A and B. The findings of multivariate analyses were as follows: Disease duration (odds ratio: 0.942, 95% CI: 0.902-0.984), smoking history (odds ratio: 2.272, 1.064-4.850), TJC (odds ratio: 0.899, 0.827-0.977), PHGA (odds ratio: 1.029, 1.012-1.047) and ESR (odds ratio: 1.015, 1.001-1.030) (Table 2).
| Achieved SDAI 50 | Did not achieve SDAI 50 | P | ||
|---|---|---|---|---|
| (Group A) | (Group B) | |||
| n | 139 | 102 | ||
| Age (years) | 60 (43-68) | 57 (46-68) | 0.946* | |
| Sex (female, %) | 82 | 85 | 0.616** | |
| Disease duration (year) | 4 (2-9) | 4 (1-11) | 0.370* | |
| Smoking history (yes, %) | 22 | 35 | 0.038** | |
| Body mass index | 21 (20-24) | 21 (19-24) | 0.537* | |
| Biological agent (naïve, %) | 78 | 68 | 0.083** | |
| Steroid dosage (mg/d) | 2 (0-5) | 2 (0-5) | 0.905* | |
| MTX dosage (mg/w) | 8 (6-10) | 8 (6-12) | 0.105* | |
| Combined csDAMRDs (yes, %) | 17 | 16 | 0.880** | |
| ESR (mm/H) | 38 (16-67) | 21 (12-51) | 0.008* | |
| CRP (mg/dL) | 1 (0.29-3.74) | 1 (0.143-2.643) | 0.053* | |
| RF (mg/dL) | 64 (18.825-197.95) n=134 | 56 '26.35-135.15) n=99 | 0.381* | |
| MMP-3 (ng/mL) | 150 (74.9-312.4) n=132 | 140 (64-309) n=100 | 0.979* | |
| TJC | 7 (4-11) | 6 (2.25-10) | 0.178* | |
| SJC | 4 (2-8) | 3 (1-6) | 0.038* | |
| PTGA | 53 (31-74.5) | 55 (28-70.75) | 0.519* | |
| PHGA | 65 (32-76) | 48 (22.25-72) | 0.020* | |
| SDAI | 26 (17-35) | 18 (11-28) n=126 | 0.006* | |
| HAQ-DI | 0 (0.125-1) n=137 | 1 (0.09375-1.125) n=96 | 0.281* | |
| SF-36 | PCS | 30 (21-39) n=103 | 30 (21-40) n=80 | 0.682* |
| MCS | 50 (44-54) n=103 | 49 (44-55) n=80 | 0.791* | |
| RCS | 45 (31-56) n=103 | 45 (32-57) n=80 | 0.824* | |
csDMARD, conventional synthetic disease-modifying antirheumatic drugs
RF, rheumatoid factor
MMP-3, matrix metalloproteinase 3
SDAI, simplified disease activity index
TJC, tender joint count
SJC, swollen joint count
PTGA, patient’s global assessment
PHGA, evaluator's global assessment
HAQ-DI, health assessment questionnaire disability index
SF-36, short form-36.
PCS, physical component summary score
MCS, mental component summary score
RCS, role/social component summary score
*analysis using MannWhitney U test
**analysis using chi-squared test for independence
| Achieved SDAI 50 | Did not achieve SDAI 50 | Odds ratio (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|
| Disease duration (year) | 4 (2-9) | 4 (1-11) | 0.942 (0.902-0.984) | 0.0069 | |||
| Smoking history (yes, %) | 22 | 35 | 2.272 (1.064-4.850) | 0.0339 | |||
| TJC | 7 (4-11) | 6 (2.25-10) | 0.899 (0.827-0.977) | 0.0121 | |||
| PHGA | 65 (32-76) | 48 (22.25-72) | 1.029 (1.012-1.047)) | 0.0009 | |||
| ESR (mm/H) | 38 (16-67) | 21 (12-51) | 1.015 (1.001-1.030) | 0.0369 | |||
4. DISCUSSION
We found that RA patients with lower disease duration, no smoking history, higher TJC, PHGA and ESR at baseline are more likely to achieve SDAI 50 following bDMARD treatment.
In this study, many predictors of RA by bDMARD treatment were reported. Female sex [9, 13-15], older age [14, 16], lower DAS 28 [14, 15, 17], lower SDAI score [8], greater pain [9], lack of initial DMARD [9] and lower HAQ-DI [14, 15] were reported. Pursuing SDAI remission at month 6 is an appropriate strategy to obtain good functional and structural outcomes at month 12 [8].
Unlike SDAI 50, which only reflects a change in disease activity components, the EULAR response includes both disease activity change and a desirable disease activity state achieved, and it was expected to be less inclusive. Compared to EULAR improvement criteria using DAS 28, SDAI 50 is easy-to-calculate and easy-to-use index in daily clinical practice. More EULAR responders (who were SDAI 50 non-responders) were observed at a higher baseline disease activity range than SDAI 50 responders (who were EULAR non-responders), which suggests that the SDAI 50 is a more stringent remission measurement than the EULAR. This report also suggests that in patients with high disease activity, the response to therapy may be underestimated when it is assessed by the SDAI 50 remission measurements, whereas in patients with lower disease activity, the response may be underestimated when using the EULAR response criteria. This possibility must be considered when evaluating the response rate in studies enrolling patients with different levels of disease activity [10]. Although a minority of patients showed discordant response measures at both ends of the disease activity spectrum at baseline, the SDAI 50 response at 3 months appears to be a more significant predictor of outcomes at 6 months than the EULAR response. Although there have been reports on early RA, there are no reports on long-established RA or studies evaluating the predictors of SDAI 50 response using bDMARDs.
These data suggest that patients with high disease activity achieved SDAI 50 response measurements. The SDAI 50 response measurements at 3 months are more significantly associated with low disease activity score/remission at 6 months than are the EULAR remission measurements, implying that although it is less sensitive than the EULAR response at high disease activity, once it is achieved, the likelihood of achieving the target outcome is greater with the use of the SDAI 50 response measurements [10]. Clinicians tend to rely more on disease states than response measures for clinical decision-making in standard practice. For example, if the SDAI score improves from 24 to 12, SDAI 12 and 24 represent moderate disease activity. However, clinically improving from SDAI 24 to 12 is beneficial to patients.
Our study had some limitations. First, we did not perform a radiographic evaluation of the joints, although we are aware that a radiographic evaluation is expected to influence the Damage-HAQ-DI [18]. Although HAQ-DI contributed to functional remission according to a multiple logistic regression analysis, the modified total vdH-Sharp score did not play a role during abatacept treatment in RA [8, 19]. In addition, because radiographic evaluations were only performed in 50 patients, the subjects were analysed without the use of radiographic evaluations. Second, we used actual clinical data that accumulated over a long period, which generated a bias in the biologicals used in this study; therefore, the clinical outcomes may differ depending on the type of biological treatment. Third, the study design is not a prospective study but a retrospective one. Finally, no socioeconomic factors were included in our analysis.
CONCLUSION
In conclusion, our study demonstrated that RA patients with shorter disease duration, no smoking, and higher disease activity of RA are more likely to achieve SDAI 50 through bDMARD treatment.
AUTHOR CONTRIBUTORS
Conception and Design
All authors.
Analysis and Interpretation of Data
Yusuke Miwa, Mayu Saito and Hidekazu Furuya.
Execution of Data
Ryo Yanai and Tsuyoshi Kasama.
Final Approval of the Article
All authors.
FUNDING
This research received no specific grants from any funding agencies in the public, commercial, or non-profit sectors.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national), and with the Helsinki Declaration of 1975, as revised in 2008.
HUMAN AND ANIMAL RIGHTS
No Animals/Humans were used for studies that are base of this research.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
Yusuke Miwa received research grants from Astellas Pharma Inc., Mitsubishi Tanabe Pharma Corporation, AbbVie CK, Pfizer Japan Inc., Chugai Pharmaceutical Co., Ltd., Eizai Co., Ltd, Asahi Kasei Pharm Co., Ltd, YL Biologics Ltd., Japan Blood Products Organization, Ono Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., and Teijin Pharma Ltd. and Kikuna Memorial Hospital in Kanagawa, Japan.
Tsuyoshi Kasama received research grants from Mitsubishi Tanabe Pharma Corporation and AbbVie CK.
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Cooperation on data collection: All SHowa University in Rheumatoid Arthritis (ASHURA) group; Yajima Nobuyuki, Takeo Isozaki, Kuninobu Wakabayashi, Sakiko Isojima, Masayu Umemura, Nao Oguro, Yoko Miura, Sho Ishii, Shinya Seki, Shinichiro Nishimi, Airi Nishimi, Yuzo Ikari, Mika Kobuna, Yoichi Toyoshima, Katsunori Inagaki and Kosuke Sakurai.
Data entry and management: Hiroka Mitsuhashi and Yuko Mitamura.







