All published articles of this journal are available on ScienceDirect.
Open-label Study of Initial and Repeat Treatment Cycles of Hylan G-F 20 in Patients with Symptomatic Knee Osteoarthritis
Abstract
Objective:
To evaluate the efficacy and safety of initial and repeat treatment with hylan G-F 20 in patients with symptomatic osteoarthritis (OA) of the knee.
Methods:
A prospective, multicenter, open-label study in adult patients with symptomatic knee OA (Kellgren-Lawrence grades I-III) undergoing repeat (SC group) or initial (IC group) treatment courses (3 x 2 mL of hylan G-F 20 at weekly intervals) was conducted with a maximum follow-up of 26 weeks. Reduction of pain using the Verbal Pain Questionnaire (VPQ) and Patient Global Assessment (PTGA) scores, concomitant pain medications use, and adverse events (AEs) were evaluated.
Results:
A total of 842 patients were included (SC group, n=314; IC group, n=528), of whom 616 formed the intent-to-treat (ITT) population (SC group, n=235; IC group, n=381). Of the 462 patients with follow-up at week 26, 311 (67.3%) were defined as responders. In the ITT population, VPQ scores decreased significantly at 26 weeks (p<0.001) compared with baseline. VPQ and PTGA scores decreased significantly (p<0.001) from baseline at all time points, without any significant changes in concomitant medication use. Twenty-four treatment-related AEs (TEAEs) were reported in 2.9% of patients, with most being mild or moderate in intensity and resolving without sequelae.
Conclusion:
Initial and repeat courses of hylan G-F 20 were effective with a favorable safety profile for knee OA. The large patient population and the study’s pragmatic design suggest that these results could be replicated in routine clinical practice.
INTRODUCTION
Osteoarthritis (OA) is the most common joint disease [1, 2] characterized by chronic and progressive pain, morning stiffness, and impaired function in the affected joint [3]. In addition to these symptoms, classic signs include crepitus, restriction of movement, and bony enlargement [3]. OA of the knee could be attributed to several reasons, including a decrease in the elasticity and viscosity of synovial fluid [2, 4-6] because of a decrease in the average number of hyaluronan (HA) molecules of normal molecular weight [4-7] and/or a reduction in the concentration of HA [5, 7], especially as a result of exudation into the joint fluid [6, 7]. Loss of elastoviscosity reduces the ability of the synovial fluid to provide protection, shock absorption, lubrication, and mechanical stability to the joint structures [6].
The primary goal of knee OA treatment is the alleviation of pain, leading to an improvement in joint function and quality of life [2]. Treatment options for knee OA include conservative therapies (e.g., physical therapy, education, weight loss), pharmacological therapies (e.g., simple analgesics, nonsteroidal anti-inflammatory drugs [NSAIDs] including cyclo-oxygenase 2 inhibitors), intra-articular injections (e.g., corticosteroids, viscosupplements), and surgical intervention (e.g., osteotomy, total knee replacement) [8, 9]. Although these treatments are typically applied in this order, combination therapy may be more effective with few adverse events [7, 10].
Viscosupplementation addresses the degradation of HA in the synovial fluid of patients with OA by the addition of exogenous HA, or its derivatives, into the affected joint via intra-articular (IA) injection [7, 11]. Viscosupplementation with high-molecular-weight HA aims to increase the viscoelasticity of synovial fluid toward normal levels, decrease pain, and improve the natural protective functions of synovial fluid in the joint [7]. High-molecular-weight, cross-linked HA was shown to markedly increase viscoelasticity and decrease the shear rate in synovial fluid [11]. Viscosupplementation can be used safely and effectively along with other concomitant therapies to reduce OA pain over the long term [10]. Alleviating knee OA pain can improve patient mobility [6].
The mode of action of HA is complex [12]. Its short-term effect is believed to be based, at least partially, on the anti-hyperalgesic and anti-inflammatory effects of the elastoviscous fluid in the affected joint [12-14]. Over the long term, the restoration of joint mobility due to relief of pain is thought to restore the trans-synovial flow and, subsequently, the metabolic and rheologic homeostasis of the joint and either synthesising endogenous HA [6, 15], preventing the degradation of HA, or both [6].
Clinical evidence shows that viscosupplementation with hylan G-F 20 (Synvisc™, Genzyme, Ridgefield, NJ), which consists of hyaluronan derivatives (hylan A and hylan B) with repating disaccharide units of N-acetylaglucosamine and sodium glucuronate (elasticity at 2.5 Hz, 1111 Pa; viscosity, 25 Pa) [16], provides symptomatic pain relief with a favorable safety profile in patients with OA of the knee [17-27]. However, limited evidence exists to support repeat courses of treatment [28, 29]. Therefore, we conducted a prospective, multicenter, open-label, pragmatic study to evaluate the efficacy and safety of a second course of hylan G-F 20 in patients with symptomatic OA of the knee seen in routine clinical practice in Germany. We also compared results with patients receiving an initial course of hylan G-F 20.
METHODS
Study Design
Of the 140 German orthopaedic physicians in their own practices who were asked to participate, 54 orthopaedic physicians were able to enroll patients into 2 groups: one group (Second Course [SC] group) included patients undergoing a second treatment cycle of hylan G-F 20, and another group (Initial Course [IC] group) included patients undergoing a first treatment cycle of hylan G-F 20. The first treatment administered in the SC group was not evaluated as part of the trial. For the IC group, the initial plan was to compare the initial course with a second course of therapy (40% to 50% of patients had been expected to receive repeat treatment during the study period as warranted by a recurrence of symptoms). However, because only 3 patients received repeat treatment treatment within the IC group, this analyses were not performed.
Patient Population
To be eligible for this study, patients had to be adults (>18 years in age), appropriate for hylan G-F 20 to treat pain associated with OA of the knee (per the product information [16]), have a diagnosis of symptomatic OA of the study knee (a pain score of at least “mild” at baseline on the Verbal Pain Questionnaire [VPQ] and Kellgren-Lawrence grade I-III), be ambulatory with an active lifestyle, and be in good general health. Additionally, to be eligible for enrollment into the SC group, patients must have had a single previous hylan G-F 20 treatment cycle in the study knee more than 6 months prior to enrollment. Patients were allowed use of any concomitant pain medication and physical therapy when needed.
Exclusion criteria included any known contraindication to hylan G-F 20, joint infection or severe inflammation, skin disease or infection in the area of the injection site, or evidence of venous or lymphatic stasis in the target leg. Patients were excluded from the SC group if they had received treatment with a viscosupplement other than hylan G-F 20 at any time, had received more than one prior hylan G-F 20 treatment cycle in the target knee, or had received hylan G-F 20 treatment in any joint other than the target knee. Patients were excluded from the IC group if they had received prior treatment with any viscosupplement (including hylan G-F 20) at any time.
Eligible patients were divided into three populations for analysis: the intent-to-treat (ITT) population included all enrolled patients who received a complete treatment cycle of hylan G-F 20; the per-protocol (PP) population included all patients who received a full treatment course and completed the visit at week 26; and the safety population included all patients who received at least one injection of hylan G-F 20.
Treatment Protocol
After screening, enrollment, and receipt of written informed consent, each patient’s medical history, demographic data, and disease characteristics were recorded.
Hylan G-F 20 treatment was administered according to the manufacturer’s instructions; 3 weekly IA injections of 2 mL hylan G-F 20 (at baseline and at 2 subsequent weeks). Knee position and injection technique were at the discretion of the treating physician and were recorded. If aspiration of synovial fluid was necessary prior to injection, the volume and quality of the synovial fluid were recorded. Other routine treatment of the patients was not changed.
A follow-up visit was conducted 1 week after the third injection (week 4). Additional follow-up consultations were performed by telephone at 12 and 26 weeks after the first injection.
Efficacy
The primary efficacy end point was the response of pain relief on the VPQ with hylan G-F 20 at 26 weeks compared with baseline. Responders were defined as patients with a pain reduction of at least one category from baseline on the VPQ. Secondary efficacy end points included the improvement in OA pain on the VPQ and the Patient Global Assessment (PTGA) at all visits. Tertiary end points were the changes in VPQ, PTGA, and concomitant pain medication use at each visit versus baseline.
The VPQ was completed by all patients at baseline, and at weeks 4, 12, and 26. Patients assessed OA pain in the target knee during the last 48 hours on a 5-point scale (1=none; 2=mild; 3=moderate; 4=severe; and 5=extreme). For the change in OA pain at weeks 4, 12 and 26, patients rated any change in their OA pain at the target knee after hylan G-F 20 treatment on a 5-point scale (1=much better; 2=better; 3=no change; 4=worse; and 5=much worse).
The PTGA was completed by all patients at baseline, and at weeks 4, 12, and 26. Patients were asked to rate their overall well-being on a 5-point scale (1=very well; 2=well; 3=fair; 4=poor; and 5=very poor).
At weeks 4, 12, and 26, patients were asked to compare their usage of concomitant pain medications (e.g., paracetamol, NSAIDs) before and after treatment with hylan G-F 20 and to rate the change on a 4-point scale (1=discontinued; 2=less; 3=no change; and 4=more).
Safety
Adverse event (AE) and serious adverse event (SAE) reports were collected and recorded from time of signature of informed consent until study completion (second telephone interview [week 26] or study withdrawal) in the safety population. Description, type, date, severity, relation (if any) to study treatment, and therapeutic measures used were recorded. AEs were coded using the most recent MedDRA dictionary version available (Med-DRA Browser version 11.1).
Statistical Methods
A total sample size of 1060 patients (SC group, n=706; IC group, n=354) was required to determine the proportion of patients with therapeutic success at week 26 (expected therapeutic success rate of 55% [a conservative estimate based on clinical experience] with a precision rate of +/- 4%). With an expected dropout rate of 30%, approximately 1514 patients receiving a second injection (SDC group, n=1008; IC group, n=506) were required.
Categorical variables are presented as frequencies and percentages. Continuous variables are presented with descriptive statistics. Tests of significance were performed using the Wilcoxon test for non-normally distributed paired samples (before/after per patient) and Bowker’s test for categorical data.
The efficacy analyses were performed on the ITT and PP populations. The primary endpoint was the change in pain score (VPQ) at 26 weeks compared with baseline, analyzed using a two-sided CI for the proportion of responders at week 26. Secondary analyses included the change in pain score (VPQ) from baseline to all other visits, and comparisons from baseline of the PTGA, which were analyzed using the Wilcoxon test for non-normally distributed paired samples (before/after per patient).
All AEs were recorded and summarized by preferred terms and by the number of patients. AEs were tabulated by group and categorized by primary system organ class, preferred term, severity, and relationship to study treatment. If a patient had the same AE on multiple occasions, then that AE was counted only once for that patient. SAEs were also listed. The proportions of patients with target knee AEs were examined using a two-sided CI.
Stepwise backward logistic regression analyses were performed at baseline to identify predictive factors for treatment success. The independent variables were sex, age, body mass index (BMI; underweight [<20 kg/m2], normal [≥20 to <25 kg/m2], overweight [≥25 to <30 kg/m2], obese [≥30 to <40 kg/m2], and severely obese [≥40 kg/m2]), disease severity, OA duration, disease location (right, left, or both knees), aspiration of synovial fluid (yes/no), injection technique (medial or lateral), knee position (extended or flexed) at the first injection, and baseline VPQ score. Odds ratios and 95% CI are presented with p-values for the various parameters.
Ethics
Written, informed consent was obtained from each patient prior to enrollment, and approval of the study design was obtained from the appropriate ethics committee of the medical association Hessen.
RESULTS
Patient Disposition and Demographics
From March 2007 to March 2009, 54 physicians enrolled and gave at least one injection to a total of 842 patients (safety population), from which 226 were considered screen failures (Fig. 1). The most common reasons for screening failure were Kellgren-Lawrence disease severity not grade I-III or missing, absence of informed consent, asymptomatic patient, and knee surgery after treatment (Fig. 1). The ITT population consisted of 616 patients and the PP population consisted of 462 patients (Fig. 1). The difference between the ITT and PP populations was because of loss to follow-up (i.e., no telephone interview available at week 26).
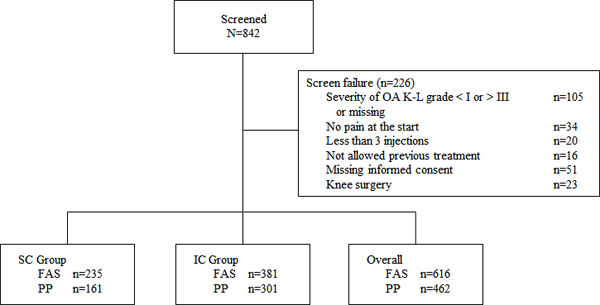
Patient demographics are shown in Table 1. More female (61.4%) than male (39.6%) patients were enrolled into the study overall, and the proportion of female patients was numerically higher in the IC group than in the SC group. In the total population, the mean age was 66.0 years (SD 11.8); age between groups was comparable. Almost three out of four patients (71.8%) were classified by BMI as overweight (≥25 to <30 kg/m2), obese (≥30 to <40 kg/m2), or severely obese (≥40 kg/m2).
| SC group (n=235) | IC group (n=381) | Total (N=616) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | N | % | |
| Sex | 235 | 100 | 381 | 100 | 616 | 100 |
| Male | 99 | 42.1 | 139 | 36.5 | 238 | 38.6 |
| Female | 136 | 57.9 | 242 | 63.5 | 378 | 61.4 |
| Age, years | 232 | 98.7 | 380 | 99.7 | 612 | 99.4 |
| <60 | 73 | 31.5 | 121 | 31.8 | 194 | 31.7 |
| 60–70 | 68 | 29.3 | 99 | 26.1 | 167 | 27.3 |
| 70–80 | 72 | 31.0 | 114 | 30.0 | 186 | 30.4 |
| >80 | 19 | 8.2 | 46 | 12.1 | 65 | 10.6 |
| BMI (kg/m2) | 229 | 97.4 | 364 | 95.5 | 593 | 96.3 |
| Underweight (<20) | 1 | 0.4 | 6 | 1.6 | 7 | 1.2 |
| Normal (≥20 to <25) | 58 | 25.3 | 102 | 28.0 | 160 | 27.0 |
| Overweight (≥25 to <30) | 111 | 48.5 | 172 | 47.3 | 283 | 47.7 |
| Obese (≥30 to <40) | 53 | 23.1 | 76 | 20.9 | 129 | 21.8 |
| Severely obese (≥40) | 6 | 2.6 | 8 | 2.2 | 14 | 2.4 |
| Knee OA location | 232 | 98.7 | 375 | 98.4 | 607 | 98.5 |
| Right | 128 | 55.2 | 185 | 49.3 | 313 | 51.6 |
| Left | 79 | 34.1 | 162 | 43.2 | 241 | 39.7 |
| Bilateral | 25 | 10.8 | 28 | 7.5 | 53 | 8.7 |
| Knee OA duration, years | 232 | 98.7 | 375 | 98.4 | 607 | 98.5 |
| <1 | 29 | 12.4 | 118 | 31.1 | 147 | 24.0 |
| 1-5 | 98 | 42.1 | 145 | 38.3 | 243 | 39.7 |
| 5-10 | 72 | 30.9 | 75 | 19.8 | 147 | 24.0 |
| >10 | 34 | 14.6 | 41 | 10.8 | 75 | 12.3 |
| Knee OA severity (Kellgren-Lawrence grade) | 235 | 100 | 381 | 100 | 616 | 100 |
| I | 23 | 9.8 | 31 | 8.1 | 54 | 8.8 |
| II | 108 | 46.0 | 147 | 38.6 | 255 | 41.4 |
| III | 104 | 44.3 | 203 | 53.3 | 307 | 49.8 |
BMI body mass index; OA osteoarthritis.
The right knee was predominantly affected by OA, and mean disease duration was 4.9 years (SD 5.5). As expected, mean disease duration was numerically longer in the SC group (5.9 years; SD 5.9) than in the IC group (4.2 years; SD 5.1). Half of patients (49.8%) were classified as Kellgren-Lawrence grade III disease severity, with 53.3% classified as grade III in the IC group and 44.3% in the SC group.
Efficacy
Verbal Pain Questionnaire
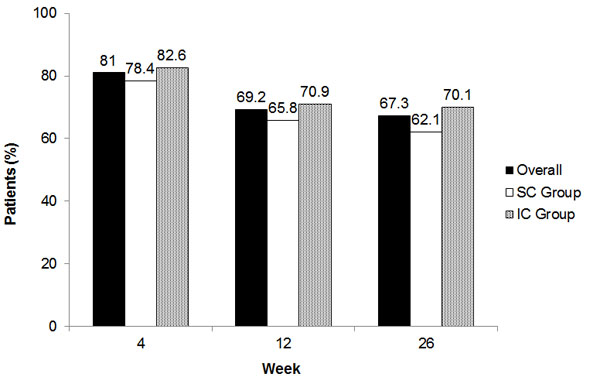
The percentage of responders was the same for the ITT and PP populations, as those populations were similar at week 26. Of the 462 patients in the PP population, 311 (67.3%; 95% CI, 63.0% to 71.6%) were defined as responders, meaning that the pain score at week 26 was reduced by at least one category in the VPQ compared with baseline (Fig. 2). This proportion was numerically higher in the IC group (70.1%) than in the SC group (62.1%; Fig. 2). At earlier weeks, the proportion of responders was higher overall and for each group than at week 26, and was numerically higher for the IC group than the SC group (Fig. 2).
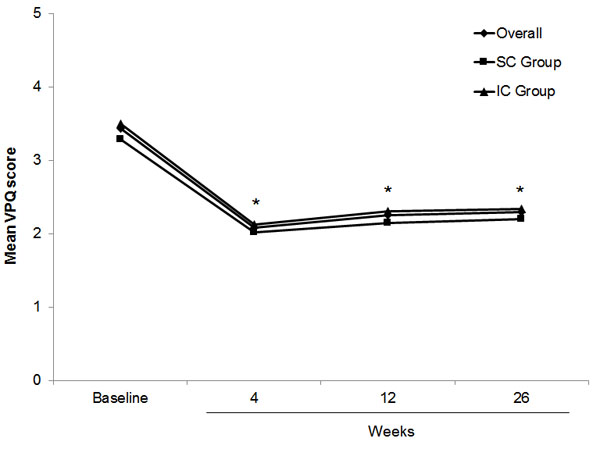
A statistically significant reduction in pain scores (VPQ) at 26 weeks was found (p<0.001) compared with baseline in the ITT patient population. The mean overall VPQ score decreased significantly from 3.38 at baseline to 2.08 at week 4 (Fig. 3). Although the mean pain score then rose slowly over time, the mean VPQ score was still significantly lower (2.33) at 26 weeks versus baseline (p<0.001). The differences in pain scores (VPQ) from baseline both overall and by treatment group were significant at all visits.
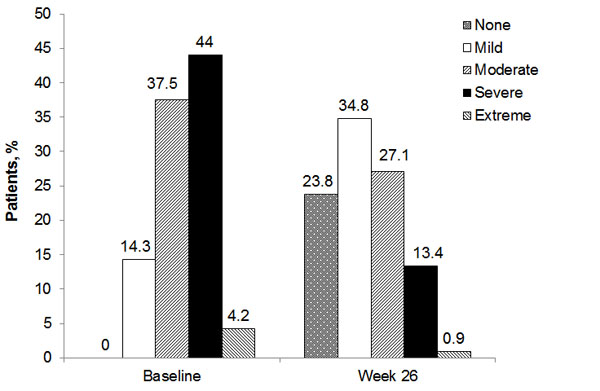
The proportions of patients within VPQ categories from baseline to week 26 are shown in Fig. (4). While all patients had some pain at baseline, 23.8% did not have any pain 26 weeks after hylan G-F 20 treatment. Almost half of patients (48.2%) rated their pain as severe or extreme at baseline while only 14.3% rated their pain that strongly at 26 weeks.
The mean PP population VPQ scores over time confirmed the results observed in the ITT population, with a considerable decrease between baseline (3.41) and week 4 (2.07), followed by a slight increase until week 26 (2.33). All results were significant versus baseline (p<0.001).
Patient Global Assessment
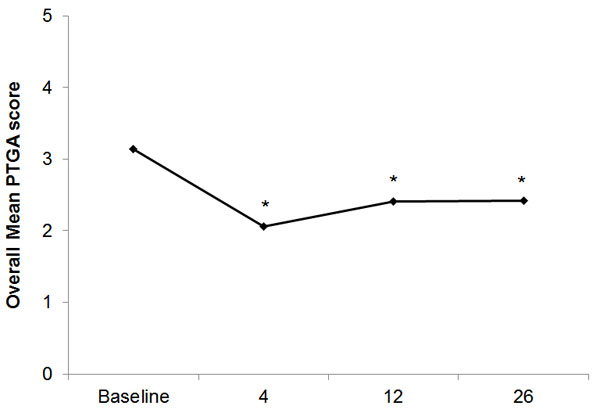
Similar to the VPQ results, a significant reduction in mean PTGA scores from 3.14 at baseline to 2.06 at week 4 (p<0.001) was observed, and the decline was still significant at 26 weeks (2.42; p<0.001; Fig. 5). More patients in the IC group numerically had improvement (59.0%) than in the SC group (52.2%) at 26 weeks.
Concomitant Medication
There were no significant changes in the use of concomitant medications during the 26-week study follow up. Among the patients evaluated at week 4, 111 (18.0%) discontinued adjuvant medication, 105 (17.0%) reduced their medication, and only 10 patients (1.6%) needed additional medication versus baseline. Discontinuation and reduction rates were higher in the IC group (21.3%, 16.8%) than in the SC group (12.8%, 17.4%). Similarly, at week 12 more patients in the IC group were able to discontinue (12.0%) or reduce (4.5%) compared with the SC group (9.6% discontinued, 2.1% reduced). Only 23 patients (5.1%) required additional pain medications at week 12. At week 26, 14 patients (3.0%) reported an increased use of concomitant pain medications. Most patients (88.7%) had no change in pain medication at week 26. Thirty-two patients (6.9%) discontinued adjuvant medication; 6 (1.3%) reported taking less pain medication at week 26. Only marginal differences between groups were found at this time.
Predictors of Treatment Success
With the stepwise backward logistic regression analysis to identify predictive factors for treatment success, three parameters, including the VPQ baseline value (p<0.001; odds ratio: 3.03; 95% CI: 2.24 to 4.11), aspiration of synovial fluid (p=0.012; odds ratio: 0.405; 95% CI: 0.20 to 0.82), and age (p=0.014; odds ratio: 0.75; 95% CI: 0.60 to 0.94), had a significant influence on treatment outcome.
Patients with the highest degree of pain at baseline experienced the greatest relief of pain during treatment. Success rates for mild, moderate, severe, and extreme pain from baseline to week 4 using VPQ scores were 34.8%, 59.7%, 79.9%, and 100% (p<0.001; chi-square test), respectively.
Patients who did not have synovial fluid aspirated prior to injection of hylan G-F 20 achieved a higher treatment success rate (287 of 418 patients; 68.7%) than those patients who underwent joint aspiration (24 of 44 patients; 54.5%). However, the number of patients with effusion and therefore undergoing joint aspiration was small (10.4%, 7.5%, and 5.4% at the first, second, and third injections, respectively), and no significant differences between these rates were found (p=0.058; chi-square test).
Although age had a significant influence by regression analysis, the success rates related to age did not show a clear trend and were not significant by chi-square testing (p=0.468). Patients aged <60 years or >80 years achieved higher success rates (72.5% and 68.0%, respectively) than patients aged 60-70 years (64.1%) and patients 70-80 years (65.5%).
Other parameters that were not statistically significant but demonstrated a trending pattern were the influence of BMI and time since OA diagnosis. More normal-weight patients (73.9%) experienced a decrease in pain compared with 68.2% of overweight and 66.3% of obese patients. Patients who were diagnosed <1 year ago had a success rate of 71.8%, 1 to 5 years ago 69.3%, 5 to 10 years ago 66.1%, and more than 10 years ago, 56.6%, respectively.
Safety and Tolerability
Overall, 842 patients (the safety population) received 2497 injections of hylan G-F 20. A total of 36 AEs were reported by 22 of 842 patients (2.6%) at week 4. Eleven patients (11/314; 3.5%) in the SC group and 11 patients (11/528; 2.1%) in the IC group experienced AEs.
A total of 24 AEs (66.7%) that were considered related to hylan G-F 20 (treatment-related AEs; Table 2). The overall incidence of treatment-related AEs was 2.9% of patients (SC group, 2.5%; IC group, 3.0%). Most treatment-related AEs were local to the treated knee (mostly pain and/or swelling and/or effusion; Table 2). The majority (n=18, 75%) were of mild or moderate intensity, and 6 were of severe intensity. Most (n=19; 79.1%) resolved without sequelae. Physicians reported 2 serious AEs (one patient each for vascular occlusion and meniscal lesion), which were considered not related to treatment. At 12 weeks (phone interview), one death and one use of a knee splint considered a serious AE were reported, however, they were not considered related to treatment.
| n | % | |
|---|---|---|
| Treatment-related AEs | 24 | 100 |
| Preferred term | ||
| Arthralgia | 6 | 25.0 |
| Hypersensitivity | 2 | 8.3 |
| Injection site joint swelling | 6 | 25.0 |
| Joint effusion | 8 | 33.3 |
| Sensation of pressure | 2 | 8.3 |
| Intensity | ||
| Mild | 3 | 12.5 |
| Moderate | 15 | 62.5 |
| Severe | 6 | 25.0 |
| Outcome | ||
| Recovered without sequelae | 19 | 79.2 |
| Symptoms resolved with treatment | 2 | 8.3 |
| Symptoms persist, no treatment | 1 | 4.2 |
| No information | 2 | 8.3 |
Severely obese patients (BMI ≥40 kg/m2) experienced the highest incidence of AEs (6.7%), compared with obese (≥30 to <40 kg/m2; no AEs), overweight (≥25 to <30 kg/m2; 1.1%), normal-weight (≥20 to <25 kg/m2; 3.1%), and underweight (<20 kg/m2; no AEs) patients.
DISCUSSION
This multicenter, prospective, open-label study conducted in routine clinical practice in Germany showed that initial and repeat courses of hylan G-F 20 are effective and well-tolerated therapeutic options in patients with symptomatic OA of the knee. Because of its pragmatic design and “real-world” setting, this study reports results that physicians might expect to replicate in their own practices.
The effectiveness of hylan G-F 20 was demonstrated by a statistically significant reduction in mean pain scores (VPQ) at all time points (p<0.001 vs. baseline) in the ITT population, and a 67.3% rate of patients indicating a response in pain relief (defined as a reduction of at least one category in the VPQ) at 26 weeks. No significant differences in effectiveness were found between patients who underwent a first or a repeat treatment cycle of hylan G-F 20, although more patients who received an initial treatment cycle achieved therapeutic success than patients receiving a second treatment cycle.
The baseline VPQ value (p<0.001), aspiration of synovial fluid (p=0.012), and age (p=0.014) had a significant influence on treatment outcome and were predictive factors for long-term therapeutic success. Even though the regression analysis showed a significant impact of synovial fluid aspiration and age, the results are difficult to interpret because of small group numbers and lack of a clear trend, respectively, and chi-square testing of the response rates was not significant. We did find, however, that the higher the pain score reported by patients at baseline, the greater the likelihood of their treatment success. These findings are consistent with those of Kemper et al. [30] who reported that patients with severe baseline pain had a greater response to treatment, likely because patients suffering from more severe pain have a higher potential for experiencing a reduction in pain than others with less severe pain.
Our patients were predominantly female and overweight or obese, which is consistent with known risk factors for knee OA, including age >50 years, female sex, and high BMI [3]. We report here a nonsignificant pattern for more normal-weight patients experiencing a decrease in pain versus overweight and obese patients. Although not significant, these results are similar to those of Kemper et al. who found that severely obese patients were significantly less likely than those of normal weight (and underweight patients more likely) to have pain reduction (p=0.04) [30]. Decreased pain was also seen more often in patients who were more (versus less) recently diagnosed with OA of the knee, although this observation was not statistically significant. Similarly, Kemper and colleagues found that patients diagnosed <1 to 5 years ago were significantly more likely to have reduced pain than those diagnosed >10 years ago (p<0.01) [30].
Concomitant pain medication was allowed during this study. At all follow-up visits (weeks 4, 12 and 26), a higher percentage of patients reported using less or discontinuing concomitant pain medication than those reporting needing additional pain medication.
No treatment-related SAEs or unexpected treatment-related AEs were reported. A total of 24 treatment-related AEs were reported (2.9% of patients). This rate is not higher than that reported in the study by Kemper et al. [30], which noted treatment-related AEs in 4.2% of patients among a population of 4253 patients. Likewise, in an open-label, 4-week substudy of repeat hylan G-F 20 injection (after 26 weeks of treatment) in 77 patients, treatment or procedure-related AE incidence was 6.5% [21].
Treatment-related AEs were reported in 2.5% of patients (0.9% of injections) in the SC group, which was comparable to the 3.0% rate (1.0% of injections) in the IC group. Similarly, Raynauld and colleagues found no difference in local AE incidence in patients receiving a first or second course of hylan G-F 20 when added to an appropriate care regimen for treating knee OA [31]. However, this is in contrast to reported AE rates in retreated patients of 18.3% and 13.1% in other studies [28, 29], which were higher than the incidences from a first course [29]. Those studies may have recruited patients with more advanced OA and more significant joint deterioration, which may increase the possibility of experiencing an AE; however, no evidence supports this idea. Here, BMI classification was identified as the only risk factor for treatment-related AEs, with severely obese patients (>40 kg/m2) having the highest risk of experiencing an AE, although a clear trend was not apparent for other BMI groups. In the study by Kemper, BMI was not a risk factor for AEs; significant predictors of AEs were age <70 years, previous HA treatment, and longer time since diagnosis [30].
This study was limited by the lack of a control group and a failure to recruit adequate numbers of patients to achieve the planned sample sizes. The results of the regression analyses should be interpreted with caution because of the uncontrolled nature of the study and the small patient subgroups. Data interpretation is also limited by potential selection bias, as only responders to first injection are more likely to receive a second injection. In addition, patients included in the repeat group did not have their initial treatment course evaluated as part of the current study, so these patients’ responses could not be compared within patient. This study also included patients with mild pain, which may interfere with the accurate assessment of hylan G-F 20; however only 14% of patients had mild pain at baseline.
Despite such limitations, the data from this study are also consistent with previously published data (RCT and real-world analyses [17-29, 32, 33]) for hylan G-F 20. In contrast to RCTs in which strict inclusion and exclusion criteria limit the types of patients treated, this study’s prospective, open-label design with a large sample size of patients provides clinically meaningful real-world information on the efficacy and safety of hylan G-F 20, and the results achieved in this study may likely be replicated by physicians at their own clinical practices.
CONCLUSION
Hylan G-F 20 has been demonstrated to be safe and effective at all disease stages [34]; as an adjunct to, or replacement for, other pharmaceutical therapies [18, 35]; and as a means of postponing or negating the need for total knee replacement when used in late-stage patients [36]. The latter finding is especially relevant given the medical contraindications and morbidity and mortality associated with joint replacement [36].
Because of the chronic nature of OA disease, interventions for OA often need to be repeated or maintained over significant periods of time, replacing or complementing other treatment modalities as required, to optimally manage patients’ symptoms. The similarity of effectiveness and safety results reported in the initial and repeat treatment groups in this study adds further weight to the evidence supporting the effectiveness and safety of hylan G-F 20 for repeat treatment in the knee joint [28, 29] and supports its inclusion in the treatment algorithm for OA.
Consistent with previously published RCT and real-world data for hylan G-F 20, these results suggest that hylan G-F 20 is safe and effective in patients with symptomatic OA of the knee and that a second course of therapy likely offers a safety profile and effectiveness comparable to an initial treatment cycle.
CONFLICT OF INTEREST
The study, data analysis, reporting, and writing of this study were sponsored by Genzyme Biosurgery GmbH. Drs. Heger, Paulsen, Fickert, and Kresmann received funding paid to their institutions for conduct of the study at their site. The authors declare no other conflicts of interest.
ACKNOWLEDGEMENTS
The authors are grateful for the input of Dr. François Bailleul and Dr. Axel Schulz to the design of this study and the interpretation of the results. Medical writing assistance was provided by Anna Porter, Ignite Marketing LLC, and to Laura Ninger and Disha Patel, PhD of Precise Publications LLC, and was funded by Sanofi.


