All published articles of this journal are available on ScienceDirect.
Association Study of Single Nucleotide Polymorphisms Rs4552569/Rs17095830 with Ankylosing Spondylitis in A Chinese Population
Abstract
Genetics play a key role in ankylosing spondylitis (AS). A previous genome-wide association study (GWAS) showed that rs4552569 (on 5q14.3) and rs17095830 (on 12q12) were associated with the risk of AS in Han Chinese, which was not replicated in other two studies. In the current study, rs4552569 and rs17095830 were genotyped in 735 Han Chinese AS patients and 1204 healthy controls using high resolution melting analysis (HRMA). We compared the distributions of genotypes and alleles between AS cases and healthy controls. Rs30187 and rs10865331, which were reported to be associated with AS susceptibility in various populations, were also genotyped and analyzed as positive controls. The results showed that no association between rs4552569/rs17095830 polymorphisms and AS susceptibility was found. On the other hand, an association between rs17095830 and one of AS complication (inflammatory bowel disease) was observed (allelic P value=0.0180; odds ratio[OR]=1.739; 95% confidence interval [CI]=1.146-2.639).
INTRODUCTION
Ankylosing spondylitis (AS), the prototypical disease in the spectrum of spondyloarthritides, is a chronic, systemic inflammation disease with a strong predilection for the axial skeleton [1-3]. It is a chronic inflammatory disease characterized by polyarticular and symmetrical arthritis affecting the hands, which often leads joint fusion and significant disability [4, 5]. AS is thought of as an autoimmune disease, though its exact pathogenesis is still poorly understood. It is well known that the disease is associated with the allele group HLA-B27. In the recent years, genome wide scans have provided some findings showing that other major histocompatibility complex (MHC) and non-MHC genes could be associated with disease susceptibility as well as phenotypic manifestations [6, 7].
In a recent GWAS report, two new susceptibility loci between EDIL3 and HAPLN1 gene at 5q14.3 (rs4552569) and within ANO6 at 12q12 (rs17095830) are associated with the risk of AS in Han Chinese [8]. However, these results were not replicated in the Taiwanese population [9]. And a more recent study also could not find the positive correlation between these two single nucleotide polymorphisms (SNPs) and risk of AS in East Asians (including Chinese, Japanese and Koreans) [10]. The reasons for these inconsistent results in similar populations are unknown. Further replication studies should be carried out to validate the association of such susceptibility loci and risk of AS in Han Chinese populations.
In the current study, we enrolled 735 AS patients and 1204 healthy controls in a Han Chinese population and assessed whether these genetic variations were associated with AS susceptibility. We further investigated the genetic association with patients’ characteristics and AS activity by using the markers rs4552569 and rs17095830. Two other SNPs, rs10865331 on intergenic DNA regions and rs30187 on ERAP1 gene, which have distinct differences of allele frequencies among different populations, were also included as positive controls. Polymorphism of rs10865331 has been reported to be closely associated with risk of AS in different populations [8, 11, 12] including Han Chinese. Rs30187, located on the exon of ERAP1 gene, is associated with risk of AS not only in Caucasians [13], but also in Han Chinese [14], Taiwanese [15] and Korean population [16].
Our results suggested that rs17095830/rs4552569 polymorphism was not associated with the AS susceptibility in Han Chinese population. Association between rs17095830 polymorphism and inflammatory bowel disease was proved.
MATERIALS AND METHODS
Patients Selection and Clinical Characteristics of as Study Cohort
735 patients fulfilling 1984 modified New York criteria for AS were recruited in a Han Chinese population. AS diagnosed by a qualified rheumatologist and sacroilitis was confirmed by a qualified radiologist. These patients were solicited sequentially at Peking University Shenzhen Hospital, Shenzhen, Guangdong, China. We collected the informed consent from the respondents before getting any clinical data. A list of the detailed clinical history including extraspinal manifestations, age and family history was shown in Supplementary Table S1. All of AS patients in this study have definite sacroilitis. We recruited a total of 1204 healthy normal controls (HNC, 732 males and 472 females) from people undergoing medical examinations. The prevalence of HLA–B27 was not determined in the collected HNCs, for most healthy donors here had not received HLA–B27 tests. The study was approved by the Peking University Health Science Center. The design of the work and final report conformed to the Declaration of Helsinki. All the subjects gave the written consent form. All the clinical parameters of patients were evaluated at first diagnosis.
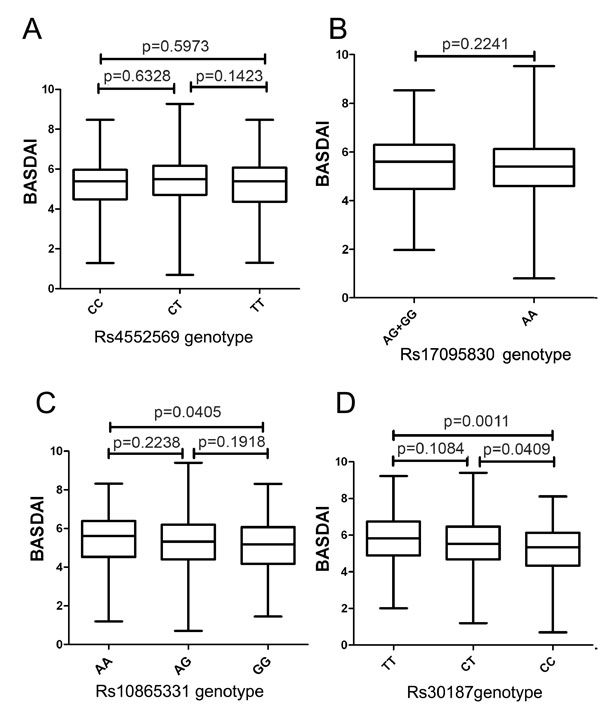
Boxplots of BASDAI measured with median showing; box: 25th-75th percentile; bars: largest and smallest values within 1.5 box lengths. A) BASDAI in groups with different genotypes of rs4552569. B) BASDAI in groups with different genotypes of 17095830. C) BASDAI in groups with different genotypes of rs10865331. D) BASDAI in groups with different genotypes of rs30187.
Bath Ankylosing Spondylitis Indices
We applied Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) to evaluate the disease activity and physical function of the AS patients enrolled. All the BASDAI and BASFI of patients were evaluated at the active stage of the disease. In the study in Taiwanese population, the modified Chinese versions of BASDAI and BASFI were shown to have good intra-class correlation with Cronbach's alpha [17].
Genotyping
Genomic DNA from the AS patients and healthy controls were isolated from peripheral blood cells using the Innogent genomic DNA extraction kit (Innogent, Shenzhen, China) following the manufacturer protocol. The genotypes of rs4552569, rs10865331, and rs30187 were directly genotyped by HRMA analysis using LightCycler software (release 1.5.0, Roche, Indianapolis, US) with high sensitivity detection and auto-grouping after polymerase chain reaction (PCR) amplification. PCR was performed in a volume of 10μl containing SsofastTM Evagreen R supermix (BIORAD, Hercules, US) 5μl, Forwardprimer (10μM) 0.5ul, Reverseprimer (10μM, Sangon, Shanghai, China) 0.5ul, Water 3ul, DNA (40ng/ul) 1ul. Rs17095830 polymorphism that could not be directly genotyped was assayed by HRMA with an unlabeled probe as previously described [18]. The sequences of the primer sets and probe for rs17095830 are listed in supplementary Table S2. The graphics of auto-grouping and sequencing of the targeted SNPs are listed in supplementary (Fig. S1).
Statistical Analysis
The constancy of Hardy-Weinberg equilibrium of the enrolled samples was first calculated. We compared the minor allele frequency between patients and controls using the chi-square test or Fisher’s exact test. Analysis of variance (ANOVA) was used to compare the mean of BASDAI index among different genotypes in AS patients. P values less than 0.05 were considered statistically significant.
RESULTS
There was no association between rs1709583/rs4552569 polymorphisms and risk of AS in the studied population
As shown in Table 1, no association between the rs4552569/rs17095830 polymorphism and AS was observed. On the other hand, for two positive control SNPs, there were significant correlations between rs30187T allele (p=0.0073; OR=1.090; 95% CI=1.024- 1.161)/rs10865331A allele (p=0.0028; OR=1.100; 95% CI=1.034-1.171) and risk of AS. In addition, no influence of age at onset, or gender on the allele frequencies of the selected polymorphisms was found (supplementary Table S3- S4). Since only a very small portion of HNCs had received HLA-B27 tests, we could only use all HNCs as the controls for the analysis of influence of HLA-B27 status. As shown in supplementary Table S5, no positive association was observed.
Genotype and allele frequencies of indicated polymorphisms in controls and patients with as.
| Case (%) | Control (%) | Case (%) | Control (%) | Genotypic | Allelic | |||
|---|---|---|---|---|---|---|---|---|
| Genotype | (n =735) | (n =1204) | Allele | (n =735) | (n =1204) | P value | P value | |
| rs4552569 | CC | 68 (9.1) | 103 (8.6) | C | 471 (32.0) | 735 (30.5) | 0.5878 | 0.3219 |
| CT | 335 (45.6) | 529 (43.9) | T | 999 (68.0) | 1673 (69.5) | |||
| TT | 332 (45.3) | 572 (47.5) | ||||||
| Hardy-Weinberg test P value | 0.4503 | 0.4598 | OR (95%CI): 1.05 (0.954-1.155) | |||||
| rs17095830 | GG | 9 (1.2) | 11 (0.9) | G | 181 (12.3) | 263 (10.9) | 0.4052 | 0.2048 |
| AG | 163 (22.2) | 241 (20.0) | A | 1289 (87.9) | 2145 (89.1) | |||
| AA | 563 (76.6) | 952 (79.1) | ||||||
| Hardy-Weinberg test P value | 0.7649 | 0.6090 | OR (95%CI): 1.127 (0.944-1.347) | |||||
| rs10865331 | AA | 216 (29.4) | 292 (24.3) | A | 796 (54.1) | 1185 (49.2) | 0.0118 | 0.0028 |
| AG | 364 (49.5) | 601 (49.9) | G | 674 (45.9) | 1223 (50.8) | |||
| GG | 155 (21.1) | 311 (25.8) | ||||||
| Hardy-Weinberg test P value | 0.9974 | 0. 9988 | OR (95%CI): 1.100 (1.034-1.171) | |||||
| rs30187 | TT | 209 (28.3) | 296 (24.6) | T | 788 (53.6) | 1184 (49.2) | 0.0222 | 0.0073 |
| CT | 370 (50.6) | 592 (49.2) | C | 682 (46.4) | 1224 (50.8) | |||
| CC | 156 (21.1) | 316 (26.2) | ||||||
| Hardy-Weinberg test P value | 0.8931 | 0.8515 | OR (95%CI): 1.090 (1.024-1.161) | |||||
AS: ankylosing spondylitis; OR: odds ratio; CI: confidence interval.
An association between rs17095830 and the inflammatory bowel disease was found
It has been reported that rs17095830 is associated with inflammatory bowel disease (IBD) in AS patients in a previous study [9]. We next investigated whether rs4552569/rs17095830 genetic polymorphisms were associated with complications (uveitis or IBD) of AS. As shown in Table 2, an association between polymorphism rs17095830 and IBD was found (allelic P value=0.0180; OR=1.739; 95%CI=1.146-2.639), which was consistent with the finding in the Taiwan population. We failed to find any association between rs4552569/rs17095830 polymorphism and uveitis (supplementary Table S6).
Genotype and allele frequencies of rs4552569 and rs17095830 in as patients with or without inflammatory bowel disease (IBD).
| IBD (%) | Without (%) | IBD (%) | Without (%) | Genotype | Allelic | |||
|---|---|---|---|---|---|---|---|---|
| Genotype | (n =49) | (n =686) | Allele | (n =49) | (n =686) | P value | P value | |
| CC | 7 (14.3) | 61 (8.9) | C | 37 (37.8) | 434 (31.6) | 0.3801 | 0.2095 | |
| rs4552569 | CT | 23 (46.9) | 312 (45.5) | T | 61 (62.2) | 938 (68.4) | ||
| TT | 19 (38.8) | 313 (45.6) | OR (95%CI): 1.194 (0.915-1.557) | |||||
| GG | 2 (2.4) | 7 (1.0) | G | 20 (20.4) | 161 (11.7) | 0.0258 | 0.0180 | |
| rs17095830 | AG | 16 (35.7) | 147 (21.0) | A | 78 (79.6) | 1211 (88.3) | ||
| AA | 31 (61.9) | 532 (78.0) | OR (95%CI): 1.739 (1.146-2.639) | |||||
AS: ankylosing spondylitis; OR: odds ratio; CI: confidence interval.
No association between rs4552569/rs17095830 polymorphism and disease severity
Here, we also investigated whether these four genetic polymorphisms are associated with disease activity. As shown in Figs. (1 and 2) and Table 3, no association between rs4552569/rs17095830 polymorphism and BASDAI or BASFI in AS patients was observed. On the other hand, rs30187T allele showed a marked correlation with BASDAI and BASFI in AS patients (Table 3). A statistical difference (P=0.0405, Fig. 1) of BASDAI between patients with AA and GG genotypes at rs10865331 was also observed (Fig 1).
Comparation of BASDAI and BASFI in as patients with different genotypes.
| rs4552569 | rs17095830 | rs10865331 | rs30187 | |||||
|---|---|---|---|---|---|---|---|---|
| Genotype (number of cases) |
CC+CT(403) | TT(332) | GG+GA(172) | AA (563) | AA+AG (580) | GG (155) | TT+CT (579) | CC (156) |
| BASDAI | 5.19±1.28 | 5.13±1.24 | 5.25±1.29 | 5.11±1.33 | 5.24±1.24 | 5.03±1.33 | 5.23±1.28 | 4.92±1.38 |
| P value | 0.5215 | 0.2241 | 0.0656 | 0.0085 | ||||
| BASFI | 4.14±2.33 | 4.01±2.29 | 4.16±2.23 | 3.96±2.44 | 4.09±2.32 | 3.93±2.51 | 4.19±2.19 | 3.75±2.38 |
| P value | 0.4483 | 0.3516 | 0.4538 | 0.0291 | ||||
DISCUSSION
Here, we performed HRMA to identify the genotypes of targeted SNPs. HRMA had better sensitivity and specificity than denaturing high performance liquid chromatography (dHPLC) with the added advantage that some homozygous sequence alterations could be identified [19, 20]. It is sometimes hard to distinguish SNPs of A/T or G/C (like rs17095830 in this study), as these SNPs cause an almost indistinguishable melting temperature (Tm) shift (< 0.4°C). In the present study, we used the unlabeled probe melting analysis to analyze polymorphism rs17095830. In this modified HRMA technique, an approximately 30-bp C3-blocked probe is used to target the SNP of interest during the melting process. A significant Tm shift (e.g., 3–4°C) could be found even when there was only a single base pair difference. As shown in our study, we were able to discriminate all of the SNPs using direct genotyping or unlabeled probe melting analysis. In addition, DNA sequencing was also performed to confirm the obtained results. These further increase the reliability of the obtained results. Using this approach, we verified the association between AS and rs10865331/rs30187, two previously defined AS-risk SNPs (Supplementary Table S4)
Our results showed no association between rs4552569/rs17095830 and AS susceptibility (Table 1). This observation was consistent with the findings in a Taiwanese population as introduced previously. The similar results were also reported in East Asian cohort (including Chinese) by a recent high throughput analysis [10]. Since then, the allele frequencies of the studied SNPs in different studies were similar (supplementary Table S7). The reasons for the inconsistencies between these studies (including the current study) and Lin et al. findings remain unknown. On the other hand, a previously reported association of the ANO6 polymorphism rs17095830 with IBD in AS patients was replicated in our studies. This gives a possible explanation between the inconsistencies between the various studies, that enrichment for IBD in the cohort of Lin et al’s study could have explained this. Differences in geographical areas that the patients reside might be considered. To precisely identify the association between rs1709583 polymorphism and risk of AS in Han Chinese, more patients with detailed grouping by maternal birthplaces might be included in future study.

Boxplots of BASFI measured with median showing; box: 25th-75th percentile; bars: largest and smallest values within 1.5 box lengths. A) BASDFI in groups with different genotypes of rs4552569. B) BASFI in groups with different genotypes of 17095830. C) BASFI in groups with different genotypes of rs10865331. D) BASFI in groups with different genotypes of rs30187.
An increasing number of non-MHC genetic associations have been reported in the recent years. Among them, the association with ERAP1 is now known to be restricted to HLA-B27 positive disease. To determine the influence of HLA-B27 status on the allele frequencies of rs4552569 and rs17095830, we should compare HLA-B27 (+) patients and HLA-B27 (+) healthy donors. However, only 53 HNCs had the results for HLA-B27 tests, for HLA-B27 test is rarely performed in regular physical examination. Thus, we were not able to use pure HLA-B27 (+) healthy donors as the controls for the analysis, which was a weakness of this analysis. Similar situation could be found in the same association study in Twains [9].
Though no association between rs10865331/rs30187 and AS severity was observed, we found a positive correlation between rs30187T allele and BASDAI/BASFI of the AS patients. This was the first report about the association between rs30187 polymorphism and AS severity in Chinese Han population. Though allele frequency of rs30187 is distinct among Caucasians, Han Chinese and Taiwanese (supplementary Table S7), rs30187T allele appeared to be a significant risk factor for development of AS in all the studied populations. Rs30187, located in the ERAP1 gene, has been shown to be associated with ankylosing spondylitis in a large (over 1,000 Caucasian patients) study. Each T allele appears to increase the odds significantly [21, 22]. Rs30187T allele causes a significant reduction in aminopeptidase activity toward a synthetic peptide substrate as well as alterations in substrate affinity [23], which might influence the disease severity. On the other hand, rs10865331A allele also showed a potential correlation with increased BASDAI in AS patients. The underlying mechanism for the involvement of this SNP in AS initiation and progression needs to be further investigated.
In summary, rs4552569 and rs17095830 genetic polymorphisms may not be susceptibility factors for AS development in Han Chinese population. Rs17095830 polymorphism has a correlation with AS patients with IBD.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's Web site along with the published article.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by General Program of Natural Science Foundation of Guangdong Province (S201201000815 & 2015A030313754) and Fundamental Research Plan of Shenzhen City (JCYJ20120831143519567 & JCYJ20140416144209741).


