RESEARCH ARTICLE
A New Curcuma Extract (Flexofytol®) in Osteoarthritis: Results from a Belgian Real-Life Experience
Thierry Appelboom*, 1, Nathalie Maes 2, Adelin Albert 3
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 77
Last Page: 81
Publisher ID: TORJ-8-77
DOI: 10.2174/1874312901408010077
Article History:
Received Date: 9/6/2014Revision Received Date: 4/8/2014
Acceptance Date: 5/9/2014
Electronic publication date: 17 /10/2014
Collection year: 2014

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
This retrospective observational study summarizes the experiences of 820 patients treated with a new Curcuma extract (Flexofytol®, 4-6 capsules per day), for more than 6 months for various forms of painful osteoarthritis. These experiences were reported by 110 Belgian general practitioners via a questionnaire that included quality-of-life parameters for assessing patient experience. Data were submitted to an independent statistician for analysis. Within the first 6 weeks, Flexofytol® improved patient pain, articular mobility, and quality of life. Excellent tolerance was reported, and more than half of these patients were able to discontinue analgaesic and anti-inflammatory drugs. Patient satisfaction was confirmed by their decision to maintain Flexofytol® therapy for more than 6 months. These data must be confirmed with randomized controlled studies. We currently conclude that Flexofytol® which is based on a new preparation of curcumin, is as a potential neutraceutical for the care of patients complaining of joint problems, with excellent tolerance and rapid benefits for articular mobility, pain, and quality of life.
INTRODUCTION
For centuries, Curcuma has been used in Ayurvedic medicine for the treatment of digestive and painful disorders. Its indications have been enlarged to include multiple myeloma, pancreatic cancer, myelodysplasic syndromes, colon cancer, and psoriasis, among other disease conditions [1]. Investigators have isolated a series of curcuminoids containing 90% curcumin or diferuloylmethane, a polyphenolic pigment from the rhizoma of the plant with demonstrated antioxidant properties [2]. Epidemiological studies have suggested that curcumin possesses anti-inflammatory, hypolipemic, anti-thrombotic, anti-tumor, anti-diabetic, and neuroprotective properties [3]. Today, with more than 5,600 pharmacological studies, the potential applications of curcumin is documented in rheumatic, traumatic, and sport-related conditions, as well as Alzheimer’s disease, gastric ulcer, irritable colon disorder, and Crohn disease [4].
Curcumin acts at various levels of the inflammation cascade; it modulates pro-inflammatory interleukin production and decreases the action of phospholipase A2, cyclooxygenase-2, and 5-lipoxygenase. Curcumin does not affect Cox-1 activity, which contributes to its excellent tolerance. Its anti-inflammatory action is due to its modulation of NFκB (anti-catabolic activity) and other transcription factors [5]. In vitro studies have revealed a protective effect of curcumin on cartilage [6]. Taken together, these lines of evidence support the use of curcumin for the treatment of rheumatic conditions.
However, the use of curcumin is limited by its low bioavailability. After oral administration, only a limited amount of the molecule penetrates the intestinal barrier to reach the circulation and the periphery of the body; the use of the native form of curcumin is therefore limited [7] To overcome these obstacles, Tilman SA, a Belgian pharmaceutical company, patented a new curcumin formulation called Flexofytol®. This innovative and licensed galenic form of curcumin uses a very thin dispersion of curcumin in a specific excipient and in the presence of emulsifiers that facilitate penetration through the intestinal barrier. Accordingly, the administration of 42 mg of this optimized curcumin preparation in a small capsule is equal to the ingestion of 57 g of native curcumin or 1 kg of Curcuma powder [6].
This new curcumin preparation has been marketed in Belgium for more than 3 years and is one of the most frequently prescribed compounds for osteoarthritis. It is recommended for maintaining articular flexibility, preserving the joints, limiting cartilage ageing, and improving articular function, particularly for sensitive joints and tendons. Flexofytol® is registered not as a drug, but as a food supplement.
We sought to perform a real-life evaluation of patient satisfaction with Flexofytol®. The present study was performed in 2013 with the help of general practitioners, with the goal of assessing their personal experience with patients suffering various manifestations of osteoarthritis. This investigation also aimed to evaluate the potential benefits of Flexofytol® on joint function, to identify the treatment period required before clinical improvement becomes apparent, to pinpoint undesirable effects, and to determine whether better results could be achieved in some locations versus others in terms of pain severity, flexibility, and quality of life.
METHODS
Patient Information
The inclusion in this observational study (not controlled or randomized) was performed over 1 year but patients were recommended by their physicians to take Flexofytol® during 6 months with evaluation visits after 6 weeks, 3 months and 6 months of treatment ; patients were also allowed to continue or discontinue their concomitant analgesic and anti-inflammatory therapies if they considered no more necessary. The diagnosis of osteoarthritis was done by X rays by an independent radiologist. Information was collected retrospectively and anonymously from the medical files of patients, as reported by 110 general practitioners who followed 820 patients treated with Flexofytol® (739 were using 2 capsules two times daily and 81 took 2 capsules 3 times daily) to improve joint function. These patients were seen as outpatients. In order to collect a maximum amount of relevant information for this study, these physicians received explanations of the aims of this study. Ethical approval was not required. Physicians were requested to report relevant clinical parameters collected during a general outpatient visit, such as age, sex, and visit date. Physicians were asked to report patient responses regarding the effect of Flexofytol®, including locations of the affected joints, pain intensity at the various locations on a scale from 0 (no pain) to 10 (intolerable pain), flexibility of the painful joint(s) (0=bad, 10=excellent), and quality of life on a visual scale from 0 (bad) to 10 (excellent). Global evaluation of the efficacy of treatment according to the patient, the use of concomitant treatments (such as analgesic and anti-inflammatory therapies), undesirable effects, and the wish of the patient to continue or discontinue the treatment were also ascertained. Variables were reported in the medical file at baseline and after 6 weeks, 3 months, and 6 months of treatment.
Statistical Analysis
Data were expressed as mean ± standard deviation for quantitative variables and scores; categorical variables were expressed as numbers and percentages. For each patient and at each visit (baseline, 6 weeks, 3 months, and 6 months), the number of concomitant drugs was also calculated. All subjects were included in an intend-to-treat analysis. Longitudinal data such as pain, mobility, and quality of life were analyzed via the General Linear Model Method in order to follow the evolution of the mean when the value was repeated for the same subject. Differences were considered statistically significant at p<0.05.
RESULTS
General Profile of the Study
One hundred and ten general practitioners from Belgium participated in this study. In total, 820 patients suffering from osteoarthritis (36.7% men), with a mean age of 64.2 ± 12.4 years, were followed. Flexofytol® was administered at a mean dose of 2 capsules twice daily in 739 patients (90.1%); the remaining patients were treated with a different dosage (3X2 capsules daily). The majority of patients (n=563; 69.3%) suffered osteoarthritis at a single location, and (n=67; 8.3%) suffered from at least three locations. The single locations were most frequently the knee and the lower back (Table 1). Before starting Flexofytol®, most patients (85%) were treated with non-steroidal anti-inflammatory drugs (54.3%), analgesic agents (64.7 %), and/or chondroprotec-tive agents (13.4%); these compounds were combined in some patients. Preliminary analysis of the medical files revealed that most patients treated with Flexofytol® attended their regular follow-up visits: 85% at the 6-week visit, 80% at the 3-month visit, and 86% at the 6-month visit.
Patient demographics.
| Men | 296 (36.7) | ||
| Women | 511 (63.3) | ||
| Age | Mean ± standard deviation | 790 | 64.2 ± 12.4 |
| Number of painful locations |
812 | ||
| 1 | 563 (69.3) | ||
| 2 | 182 (22.4) | ||
| 3+ | 67 (8.3) | ||
| Most frequent locations |
812 | ||
| knee | 175 (21.6) | ||
| lower back | 155 (19.1) | ||
| hip | 57 (7.0) | ||
| neck | 53 (6.5) | ||
| hand (thumb + fingers) | 51 (6.3) | ||
| shoulder | 49 (6.0) | ||
| knee + back | 31 (3.8) | ||
| neck + back | 29 (3.6) | ||
| hip + hand | 28 (3.4) | ||
| knee + hip | 21 (2.6) | ||
| other | 163 (20.1) |
Reduction of concomitant therapies already occurred within 6 weeks and was sustained at 6 months.
| Time of Follow Up | Mean Number (SD) of Concomitant Therapies Per Patient |
|---|---|
| -Baseline | 1.60 (0.73) |
| -6 weeks | 0.93 (0.71) |
| -3 months | 0.92 (0.73) |
| -6 months | 0.75 (0.73) |
Effect of Flexofytol® on Clinical Parameters
Pain severity progressively and significantly declined in most patients within 6 months; the mean score fell from 6.9 to 3.2 (p<0001; Fig. 1). The patient’s impression of flexibility significantly increased from a mean score of 4.2 prior to treatment to 6.7 after 6 months of therapy (p<0.0001) (Fig. 1). This double improvement was associated with better quality of life, since the mean score increased from 4.7 to 6.9 within the same time period (p<0.0001) (Fig. 1). It is interesting to note that most of the reported improvement occurred within the first 6 weeks of therapy.
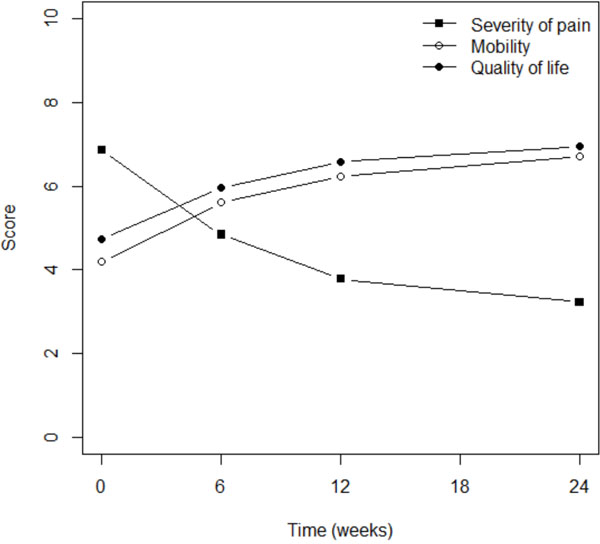 |
Fig. (1). Pain reduction was associated with better mobility and quality of life. |
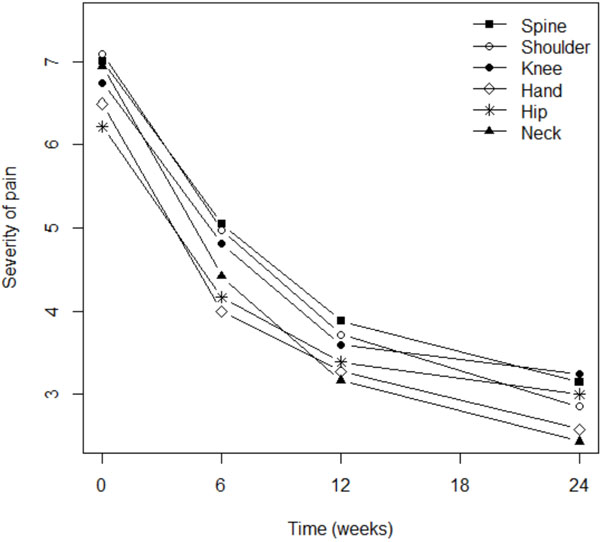 |
Fig. (2). Targets of the analgaesic effect of Flexofytol®. |
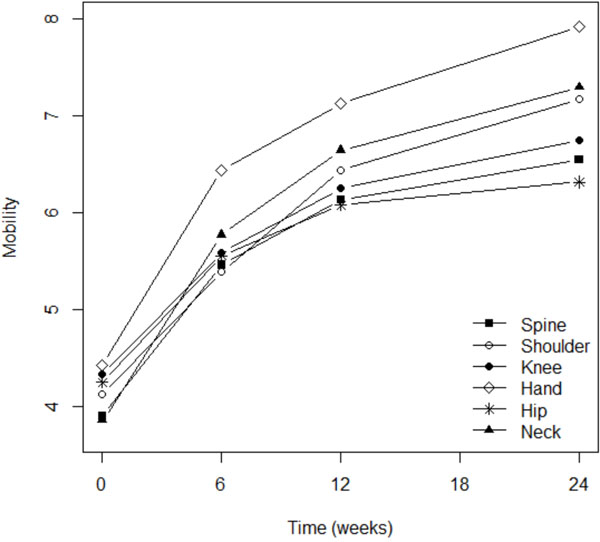 |
Fig. (3). Improvement in flexibility was more marked for the hand. |
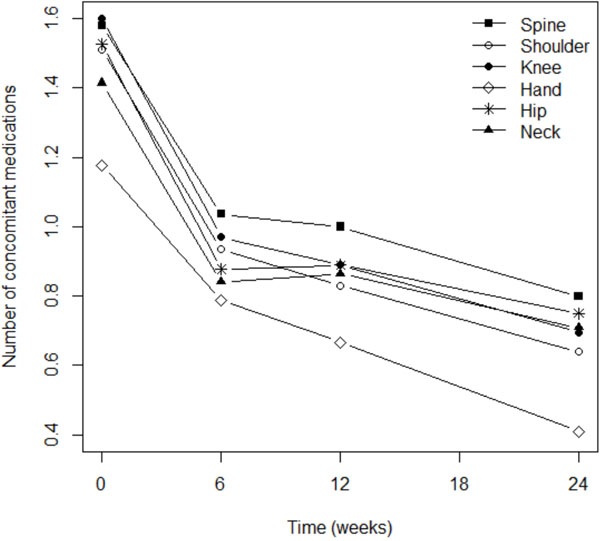 |
Fig. (4). Reductions in concomitant therapies were independent of osteoarthritis location. |
Targets of Flexofytol®
Although pain severity was more intense among patients with osteoarthritis of the knee (p=0.003), shoulder (p=0.016), or spine (p=0.0001), the analgesic effect of Flexofytol® was similarly reported in all these locations (Fig. 2). Patient perception of flexibility was less evident for the spine (p=0.023) but better for the hand (p=0.014), while clinical improvement over time was more evident for patients suffering from pain in the knee (p=0.0045) or in the hip (p=0.036; Fig. 3). Quality of life improved overt time, but less among patients suffering from low back pain (p=0.0004) or hip pain (p=0.012). This study also revealed that higher pain scores were associated with higher numbers of painful osteoarthritic lesions. More lesions were also associated with lower general evaluations of articular flexibility and quality of life. (p=0.041) (similar benefits of Flexofytol® were reported whether the patient suffered from one or more painful joints).
Reduction of Concomitant Treatments
When patients were treated with Flexofytol®, they significantly reduced the number of additional treatments they took for osteoarthritis (p<0.0001; Fig. 4). This phenomenon was independent from the locations of pain (Fig. 4). However, the consumption of concomitant therapies remained elevated among patients suffering from pain in the spine (p=0.014) or the shoulder (p=0.040). It is interesting to note that the reduction in the use of concomitant therapies was statistically significant in the first 6 weeks after the initiation of Flexofytol® therapy (Fig. 4 and Table 2). In general, Flexofytol® allowed patients to reduce the number of other treatments of osteoarthritis, independent of the number of painful locations or concomitant therapies.
Patient Global Assessments
Global assessments provided by the patients confirmed the specific data obtained at 6 weeks after therapy initiation. At 6 weeks, 77.1% of patients were already satisfied with their use of Flexofytol®, and 79% were still satisfied at 6 months. Accordingly, the percentage of satisfied patients was strikingly higher than that of patients whose pain was unchanged (18.9%) or had deteriorated (2.1%) at the end of the follow-up period. After 6 months of therapy, 41.7% of the patients who were seen at this visit wished to continue Flexofytol® for a longer period, 10.8% responded negatively, and 47.5% had no opinion for continuing for a longer period.
Undesirable Effects
During the follow-up period, side effects were reported by 3.6% of the patients at 6 weeks, by 2.5% at 3 months, and by 1.3% at 6 months. Most adverse events were related to gastrointestinal intolerance, for example diarrhoea.
DISCUSSION
In the present investigation, the experience of 110 Belgian general practitioners suggests that 2 capsules of Flexofytol® taken twice daily can significantly improve the pain, articular flexibility, and quality of life of osteoarthritic patients, nearly without undesirable effects and within 6 weeks (Fig. 1). Flexofytol® also reduced the consumption of concomitant therapies for osteoarthritis, such as anti-inflammatory drugs, analgaesic drugs, and chondroprotective agents, and was associated with a high degree of patient satisfaction (Fig. 4). The clinical improvement was maintained at least up to 6 months despite the statistical reduction of concomitant analgesic and anti-inflammatory therapies at 6 weeks suggesting that the benefit was not related to the concomitant therapies. The best indications of Flexofytol® suggested from this study are painful osteoarthritis of the knee or of the hip, although benefits were also reported in other degenerative joint conditions (Fig. 2). The weakest point of this study is that it is observational, not randomized, not double blind and not placebo controlled; yet the data come from a collection of real-life information that reflects the experiences of 110 physicians over 1 year and of 820 patients over 6 months of therapy. This approach is quite different from a randomized, controlled, double blind study with a reference molecule, but includes a large number of physicians and patients and covers a relatively long period of observation. It is also important to stress the good compliance of the treated patients and the proportion of patients who wished to continue Flexofytol® therapy longer than 6 months. The observed benefits at 6 weeks may be due to the anti-inflammatory mode of action and/or analgaesic effect of curcumin, while patient satisfaction at 6 months may result from additional regenerating effects of curcumin on articular structures. A series of in vitro studies previously uncovered an anti-catabolic effect of curcumin that inhibits metalloproteases and stimulates the production of glycosaminoglycans, which play a role in the accumulation of aggrecans in the cartilage and reinforce the matrix; curcumin also promotes the formation of chondrocytes [4, 6].
Curcumin is relatively stable in the intestine, but to detect curcumin in circulating plasma, dosages as high as 10-12 g daily are necessary. Once in the blood, curcumin can persist for a long time, and can penetrate the peripheral tissue due to its polyphenol structure [6, 7]. The curcumin extract in Flexofytol® is a micro-emulsion that is 1350 times more assimilable than native curcumin powder. In Flexofytol® capsules, curcumin is present in the centre of a 200-nm micelle constituted of polysorbate with a hydrophilic extremity on the periphery and a hydrophobic extremity oriented toward the centre. This formulation differentiates Flexofytol® from the other available preparations of curcumin, which for example use black pepper or piperidine as supports, or are combined with other plant extracts. The benefits of curcumin in osteoarthritis have been reported in other studies as well [6] The administration of 1 g Meriva (a curcumin extract coated in a phytosome to facilitate its resorption by the intestine) over 3-8 months also improved symptoms, articular function, and quality of walking; these benefits were associated with reductions in the levels of inflammation mediators such as Interleukin 1(, Interleukin 6, the ligand of CD40, and other adhesion molecules, as well as reductions in the blood sedimentation rate [8-10].
Other clinical trials with different curcumin preparations in osteoarthritis have been recently published in the literature; accordingly, a double blind prospective randomized clinical trial has suggested a benefit of curcumin as an adjuvant therapy to diclofenac (75 mg daily) in primary knee osteoarthritis but no statistical significance [11]. First in a 3 month study, Meriva (a curcumin phytosome phosphatidyl complex) decreased pain and improved joint function in 50 osteoarthritis patients. In a following study in 100 patients, Meriva also improved a series of inflammatory markers and sedimentation rate as well [12]. A curcuma domestica extract seems to be as effective as ibuprofen for the treatment of osteoarthritis but with fewer gastrointestinal adverse events [13]. A randomized double blind placebo control parallel group clinical trial conducted among patients with mild-moderate knee osteoarthritis showed a greater reduction of Womac, Visual analogue score and Lequesne pain functional index than in placebo and also on pain and physical function [14].
In rheumatoid arthritis, BCM-95, another curcumin formulation, improved the disease articular score more than the anti-inflammatory drug diclofenac, suggesting that curcumin could be considered as an alternative to non-steroidal anti-inflammatory drugs, with better tolerance; note that in animal models, curcumin exerts an analgaesic effect [15].
In conclusion, Flexofytol® like other curcumin extracts is appropriate for the care of patients complaining of joint problems. Flexofytol® may not only address painful joints, but may also contribute to the regeneration of cartilage.
CONFLICT OF INTEREST
Thierry Appelboom (University of Brussels) received a grant from Tilman SA for his participation in the protocol and revision of the text. Nathalie Maes and Adelin Albert of the Department of Medico-economic Information of the University of Liège received a grant from Tilman SA for the statistical analysis of the results.
ACKNOWLEDGEMENTS
Declared none.






